Enzyme-Assisted Extraction Optimization, Characterization and Antioxidant Activity of Polysaccharides from Sea Cucumber Phyllophorus proteus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of Polysaccharides
2.3. Experimental Design of RSM
2.4. Isolation of Homogeneous Polysaccharides from PPP
2.5. Characterization of PPP, PPP-1a, PPP-1b and PPP-2
2.5.1. Determination of Homogeneity and Molecular Weight
2.5.2. Analysis of Monosaccharide Composition, Protein and Sulfate Content
2.5.3. NMR Analysis
2.5.4. Specific Rotation, Intrinsic Viscosity and FT-IR Spectrometric Analysis
2.6. Assay of In Vitro Antioxidant Activities
2.6.1. Assay of DPPH Radical Scavenging Activity
2.6.2. Assay of Hydroxyl Radical Scavenging Activity
2.6.3. Assay of Superoxide Radical Scavenging Activity
2.6.4. Assay of ABTS Radical Scavenging Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extraction Parameters
3.1.1. Model Building and Statistical Analysis
3.1.2. Optimization of Extraction Conditions of PPP and Validation of the Model
3.2. Isolation and Characterization of Polysaccharides from PPP
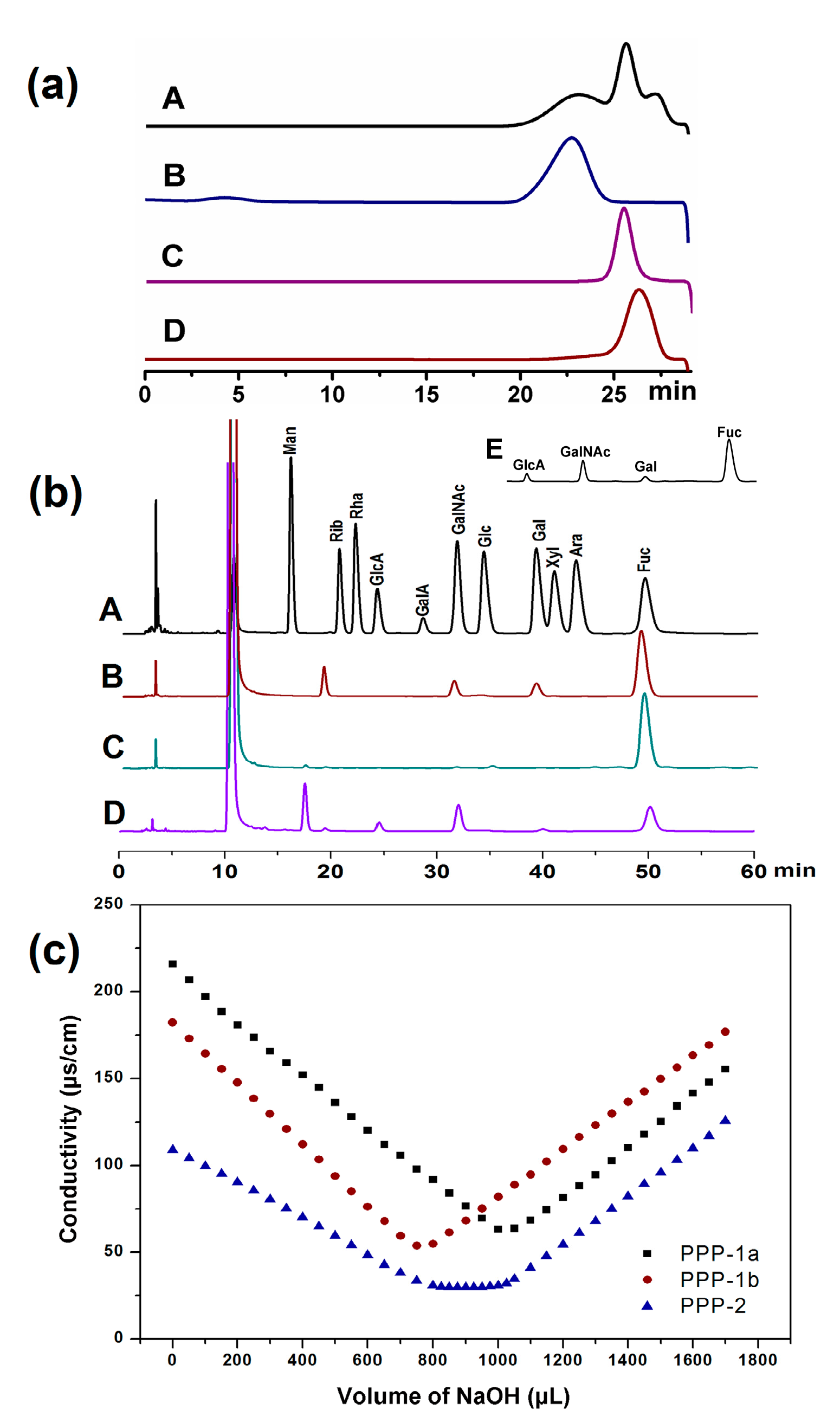
3.2.1. Homogeneity and Molecular Weights of PPP, PPP-1a, PPP-1b and PPP-2
3.2.2. Monosaccharide Composition of PPP, PPP-1a, PPP-1b and PPP-2
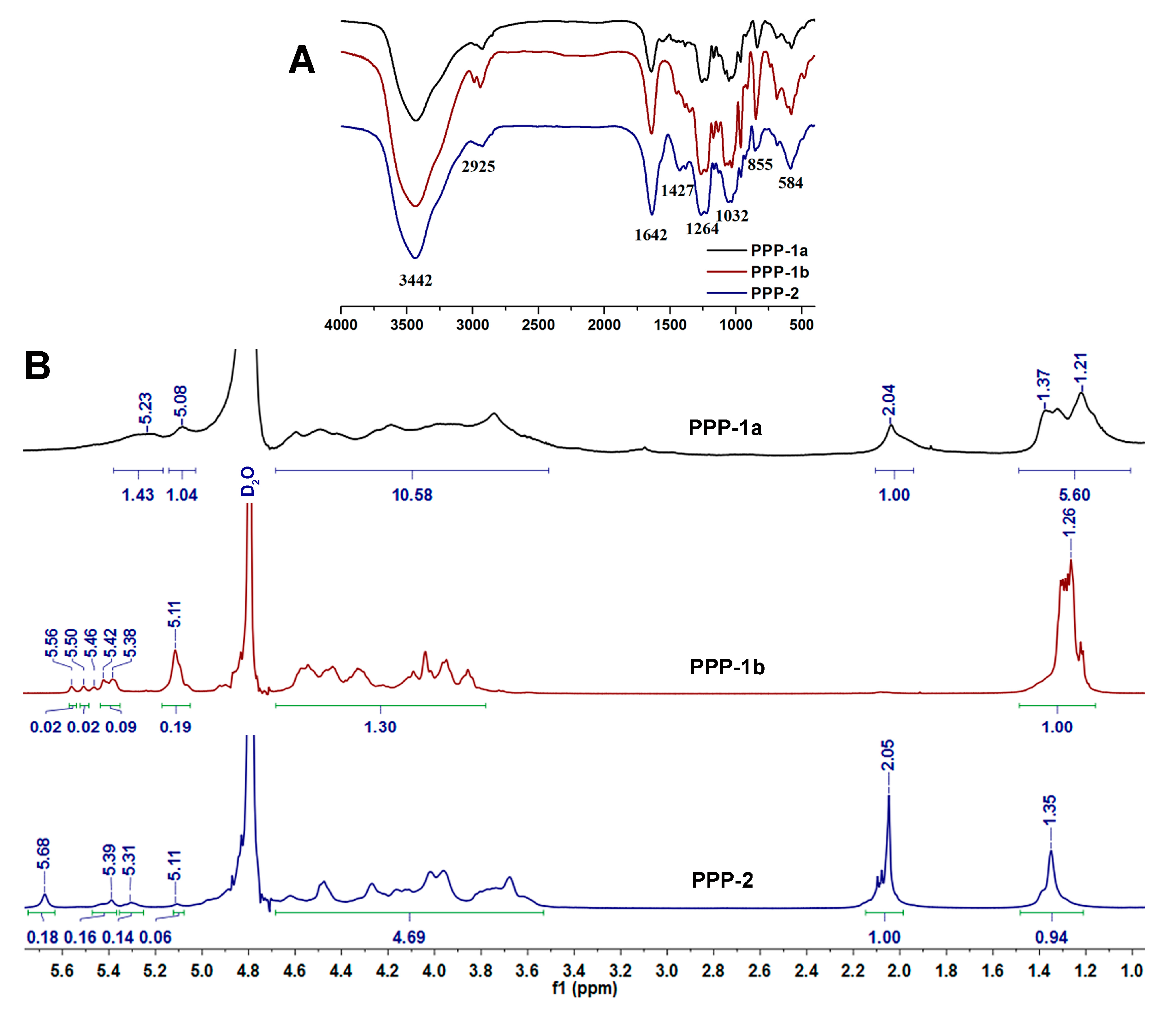
3.2.3. Specific Rotation, Intrinsic Viscosity and FT-IR Spectrometric Analysis
3.2.4. NMR Analysis of PPP-1a, PPP-1b and PPP-2
3.3. In Vitro Antioxidant Activity of PPP
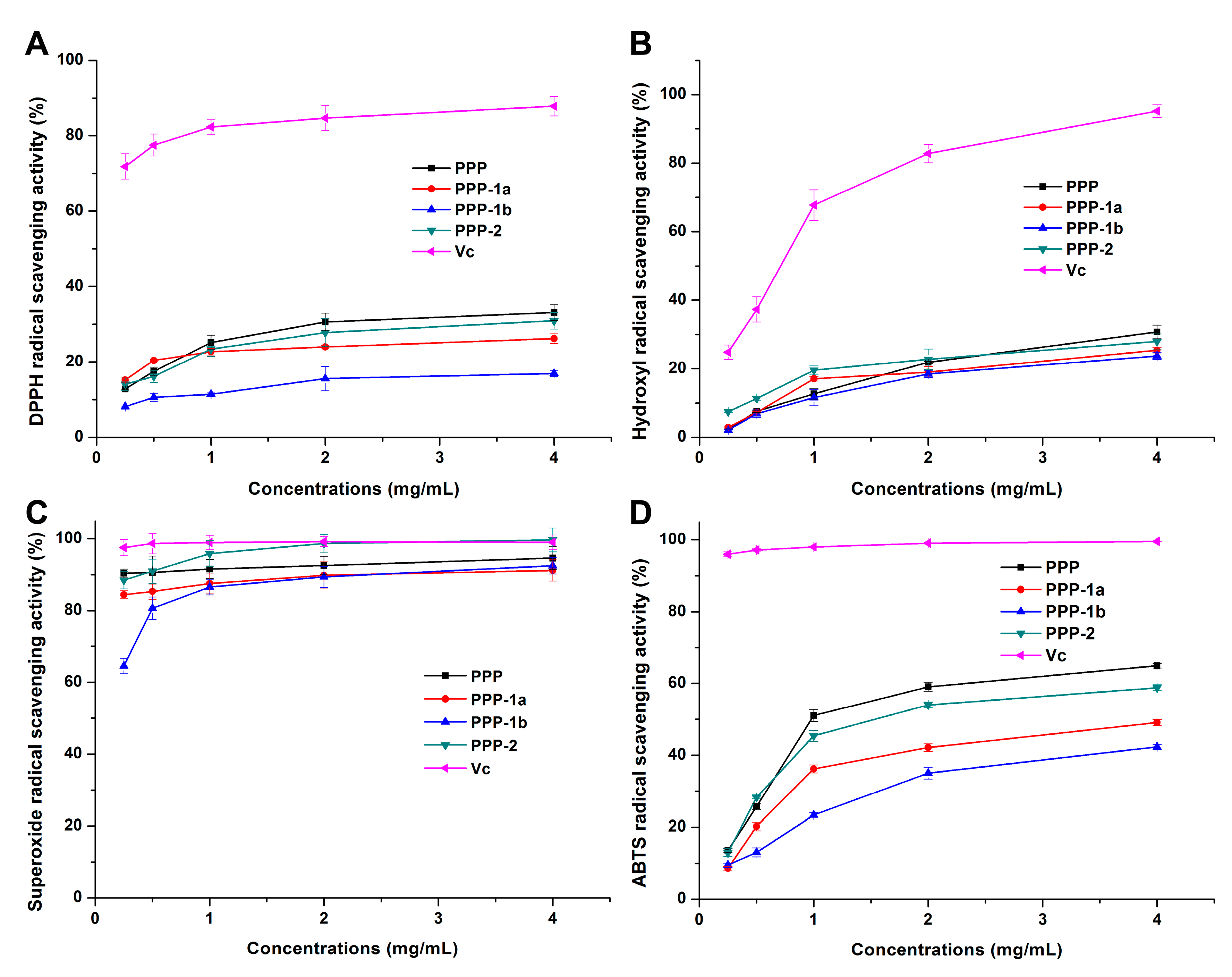
3.3.1. DPPH Radical Scavenging Activity
3.3.2. Hydroxyl Radical Scavenging Activity
3.3.3. Superoxide Radical Scavenging Activity
3.3.4. ABTS Radical Scavenging Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnes, A.T.; Quetin, L.B.; Childress, J.J.; Pawson, D.L. Deep-sea macroplanktonic sea cucumbers: suspended sediment feeders captured from deep submergence vehicle. Science 1976, 194, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Hao, J.; Zhao, X.; Lang, Y.; Fan, F.; Cai, C.; Li, G.; Zhang, L.; Yu, G. In Vivo anti-cancer mechanism of low-molecular-weight fucosylated chondroitin sulfate (LFCS) from sea cucumber Cucumaria frondosa. Molecules 2016, 21, 625. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, H.; Wang, W.; Jia, B.; Yang, T.; Zhao, Z.; Ding, J.; Xiao, X. Differentiation of dried sea cucumber products from different geographical areas by surface desorption atmospheric pressure chemical ionization mass spectrometry. J. Agric. Food Chem. 2009, 57, 9356–9364. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.A.; Surayot, U.; You, S.G. Structural characterization of immunostimulating protein-sulfated fucan complex extracted from the body wall of a sea cucumber, Stichopus japonicus. Int. J. Biol. Macromol. 2017, 99, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Liao, N.B.; Zhang, Y.; Ye, X.Q.; Li, S.; Hu, Y.Q.; Liu, D.H.; Linhardt, R.J.; Wang, X.; Chen, S.G. In vitro fermentation behaviors of fucosylated chondroitin sulfate from Pearsonothuria graeffei by human gut microflora. Int. J. Biol. Macromol. 2017, 102, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.; Kalinovsky, A.; Avilov, S.; Andryjaschenko, P.; Dmitrenok, P.; Kalinin, V.; Chingizova, E.; Minin, K.; Stonik, V. Structures and biogenesis of fallaxosides D4, D5, D6 and D7, trisulfated non-holostane triterpene glycosides from the sea cucumber Cucumaria fallax. Molecules 2016, 21, 939. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Ishida, M.; Aida, K.; Tsuduki, T.; Zhang, J.; Manabe, Y.; Hirata, T.; Sugawara, T. Dietary cerebroside from sea cucumber (Stichopus japonicus): Absorption and effects on skin barrier and cecal short-chain fatty acids. J. Agric. Food Chem. 2016, 64, 7014–7021. [Google Scholar] [CrossRef] [PubMed]
- He, L.-X.; Zhang, Z.-F.; Sun, B.; Chen, Q.-H.; Liu, R.; Ren, J.-W.; Wang, J.-B.; Li, Y. Sea cucumber (Codonopsis pilosula) oligopeptides: Immunomodulatory effects based on stimulating Th cells, cytokine secretion and antibody production. Food Funct. 2016, 7, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, L.; Zhao, L.; Xiao, C.; Gao, N.; Luo, L.; Yang, L.; Li, Z.; Chen, L.; Zhao, J. Structural analysis and anticoagulant activities of the novel sulfated fucan possessing a regular well-defined repeating unit from sea cucumber. Mar. Drugs 2015, 13, 2063–2084. [Google Scholar] [CrossRef] [PubMed]
- Myron, P.; Siddiquee, S.; Al Azad, S. Fucosylated chondroitin sulfate diversity in sea cucumbers: A review. Carbohydr. Polym. 2014, 112, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, M.; Xiao, C.; Yang, L.; Zhou, L.; Gaoa, N.; Li, Z.; Chen, J.; Chen, J.; Liu, J.; Qin, H.; Zhao, J. Discovery of an intrinsic tenase complex inhibitor: Pure nonasaccharide from fucosylated glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, Y.; Ye, X.; Li, G.; Yu, G.; Xue, C.; Chai, W. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim. Biophys. BBA Gen. Subj. 2012, 1820, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Holothurian fucosylated chondroitin sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Xie, Y.; Wang, W.; Yan, Y.; Ye, H.; Jabbar, S.; Zeng, X. Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves. Carbohydr. Polym. 2015, 128, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, G.; Zhang, J.; Zhang, G.; Jia, L.; Liu, X.; Deng, P.; Fan, K. Extraction optimization and antioxidant activity of intracellular selenium polysaccharide by Cordyceps sinensis SU-02. Carbohydr. Polym. 2011, 86, 1745–1750. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Mao, G.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Zhou, L.; Zhang, T.; Yang, J.; et al. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydr. Polym. 2014, 101, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, Y.; Ye, X.; Hu, Y.; Ding, T.; Chen, S. Sulfation pattern of fucose branches affects the anti-hyperlipidemic activities of fucosylated chondroitin sulfate. Carbohydr. Polym. 2016, 147, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Zhi, Z.; Wei, C.; Wang, W.; Ding, T.; Ye, X.; Hu, Y.; Linhardt, R.J.; Chen, S. Macromolecular properties and hypolipidemic effects of four sulfated polysaccharides from sea cucumbers. Carbohydr. Polym. 2017, 173, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Esmat, A.Y.; Said, M.M.; Soliman, A.A.; El-Masry, K.S.H.; Badiea, E.A. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition 2013, 29, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Mena-Bueno, S.; Atanasova, M.; Fernández-Trasancos, Á.; Paradela-Dobarro, B.; Bravo, S.B.; Álvarez, E.; Fernández, Á.L.; Carrera, I.; González-Juanatey, J.R.; Eiras, S. Sea cucumbers with an anti-inflammatory effect on endothelial cells and subcutaneous but not on epicardial adipose tissue. Food Funct. 2016, 7, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Z.; Zhang, M.; Meng, X.; Xia, X.; Yuan, W.; Xue, F.; Liu, C. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohydr. Polym. 2012, 90, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hu, C.; Zhang, L.; Fan, S. Genetic identification of global commercial sea cucumber species on the basis of mitochondrial DNA sequences. Food Control 2011, 22, 72–77. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Saito, H.; Yamagata, T.; Suzuki, S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J. Biol. Chem. 1968, 243, 1536–1542. [Google Scholar] [PubMed]
- Casu, B.; Gennaro, U. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr. Res. 1975, 39, 168–176. [Google Scholar] [CrossRef]
- Zhao, L.; Lai, S.; Huang, R.; Wu, M.; Gao, N.; Xu, L.; Qin, H.; Peng, W.; Zhao, J. Structure and anticoagulant activity of fucosylated glycosaminoglycan degraded by deaminative cleavage. Carbohydr. Polym. 2013, 98, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fu, S.; Zhu, X.; Zhang, L.M.; Yang, Y.; Yang, X.; Liu, H. Hyperbranched acidic polysaccharide from green tea. Biomacromolecules 2010, 11, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C. hai In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Bi, H.; Gao, T.; Li, Z.; Ji, L.; Yang, W.; Jeff Iteku, B.; Liu, E.; Zhou, Y. Structural elucidation and antioxidant activity of a water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries). Food Chem. 2013, 138, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Ibrahim, S.A.; Gao, S.S.; Yang, H.; Huang, W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016, 145, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Zhang, J. Optimization of enzyme assisted extraction of polysaccharides from Astragalus membranaceus. Carbohydr. Polym. 2014, 111, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Q.; Zhou, X.; Zeng, F.; Lu, X. Extraction of Opuntia dillenii Haw. polysaccharides and their antioxidant activities. Molecules 2016, 21, 1612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.P.; Zhai, X.C.; Li, L.Q.; Wu, X.X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Shashkov, A.S.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization of fucosylated chondroitin sulfates from sea cucumbers Apostichopus japonicus and Actinopyga mauritiana. Carbohydr. Polym. 2016, 153, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Zhao, Y.; Hu, S.; Shi, D.; Xue, C. Fucoidan from sea cucumber Cucumaria frondosa exhibits anti-hyperglycemic effects in insulin resistant mice via activating the PI3K/PKB pathway and GLUT4. J. Biosci. Bioeng. 2016, 121, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ye, X.; Sun, Y.; Wu, D.; Wu, N.; Hu, Y.; Chen, S. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J. Agric. Food Chem. 2014, 62, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, W.; Zhang, C.; Xue, C.; Chang, Y.; Wu, X.; Tang, Q.; Wang, J. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chem. 2012, 133, 1414–1419. [Google Scholar] [CrossRef]
- Yu, L.; Xue, C.; Chang, Y.; Xu, X.; Ge, L.; Liu, G.; Wang, Y. Structure elucidation of fucoidan composed of a novel tetrafucose repeating unit from sea cucumber Thelenota ananas. Food Chem. 2014, 146, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Ye, X.; Guo, X.; Liao, N.; Yin, X.; Hu, Y.; Sun, Y.; Liu, D.; Chen, S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuria graeffei, via 60Co irradiation. Carbohydr. Polym. 2013, 93, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, S.; Zhao, J.; Kang, H.; Ding, H. Physicochemical characteristics and anticoagulant activities of low molecular weight fractions by free-radical depolymerization of a fucosylated chondroitin sulphate from sea cucumber Thelenata ananas. Food Chem. 2010, 122, 716–723. [Google Scholar] [CrossRef]
- You, L.; Gao, Q.; Feng, M.; Yang, B.; Ren, J.; Gu, L.; Cui, C.; Zhao, M. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013, 138, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Kan, J.; Li, Z.; Chen, Z. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr. Polym. 2005, 61, 125–131. [Google Scholar] [CrossRef]
- Ding, X.; Feng, S.; Cao, M.; Li, M.T.; Tang, J.; Guo, C.X.; Zhang, J.; Sun, Q.; Yang, Z.R.; Zhao, J. Structure characterization of polysaccharide isolated from the fruiting bodies of Tricholoma matsutake. Carbohydr. Polym. 2010, 81, 942–947. [Google Scholar] [CrossRef]
- Yoshida, K.-I.; Minami, Y.; Nemoto, H.; Numata, K.; Yamanaka, E. Structure of DHG, a depolymerized glycosaminoglycan from sea cucumber, Stichopus japonicus. Tetrahedron Lett. 1992, 33, 4959–4962. [Google Scholar] [CrossRef]
- Panagos, C.G.; Thomson, D.S.; Moss, C.; Hughes, A.D.; Kelly, M.S.; Liu, Y.; Chai, W.; Venkatasamy, R.; Spina, D.; Page, C.P.; et al. Fucosylated chondroitin sulfates from the body wall of the sea cucumber Holothuria forskali: Conformation, selectin binding, and biological activity. J. Biol. Chem. 2014, 289, 28284–28298. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Ribeiro, A.C.; Alves, A.P.; Vieira, R.P.; Mourão, P.A.S. Sulfated fucans from echinoderms have a regular tetrasaccharide repeating unit defined by specific patterns of sulfation at the O-2 and O-4 positions. J. Biol. Chem. 1994, 269, 22113–22123. [Google Scholar] [PubMed]
- Tako, M.; Nakada, T.; Hongou, F. Chemical characterization of fucoidan from commercially cultured Nemacystus decipiens (Itomozuku). Biosci. Biotechnol. Biochem. 1999, 63, 1813–1815. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C. Review: Methods used to evaluate the free radical sacavening activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, S.K.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Zhang, Q.; Zhang, Z.; Qi, H.; Li, P. Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chem. 2009, 114, 1285–1290. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chang, J.; Zhang, L.; Zhang, J.; Li, S. Purification and antioxidant activity of a polysaccharide from bulbs of Fritillaria ussuriensis Maxim. Int. J. Biol. Macromol. 2012, 50, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, C.; Xu, Z.; Wang, Y. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 2007, 42, 961–970. [Google Scholar]
- Jin, L.; Guan, X.; Liu, W.; Zhang, X.; Yan, W.; Yao, W.; Gao, X. Characterization and antioxidant activity of a polysaccharide extracted from Sarcandra glabra. Carbohydr. Polym. 2012, 90, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, J.C.; Kennedy, J.F. Purification, composition analysis and antioxidant activity of different polysaccharide conjugates (APPs) from the fruiting bodies of Auricularia polytricha. Carbohydr. Polym. 2010, 82, 299–304. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, Y.; Guan, R.; Zheng, W.; Liu, J.; Zhao, J. Digestibility of fucosylated glycosaminoglycan from sea cucumber and its effects on digestive enzymes under simulated salivary and gastrointestinal conditions. Carbohydr. Polym. 2018, 186, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Pan, R.; Dong, X.; He, M.; Wang, C. Physicochemical properties and antioxidant activities of two fucosylated chondroitin sulfate from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2016, 51, 650–658. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, H.; Liu, W.; Pei, J.; Wang, Z.; Zhou, H.; Yan, J. Ultrasound enhanced production and antioxidant activity of polysaccharides from mycelial fermentation of Phellinus igniarius. Carbohydr. Polym. 2014, 113, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds PPP, PPP-1a, PPP-1b and PPP-2 are available from the authors. |
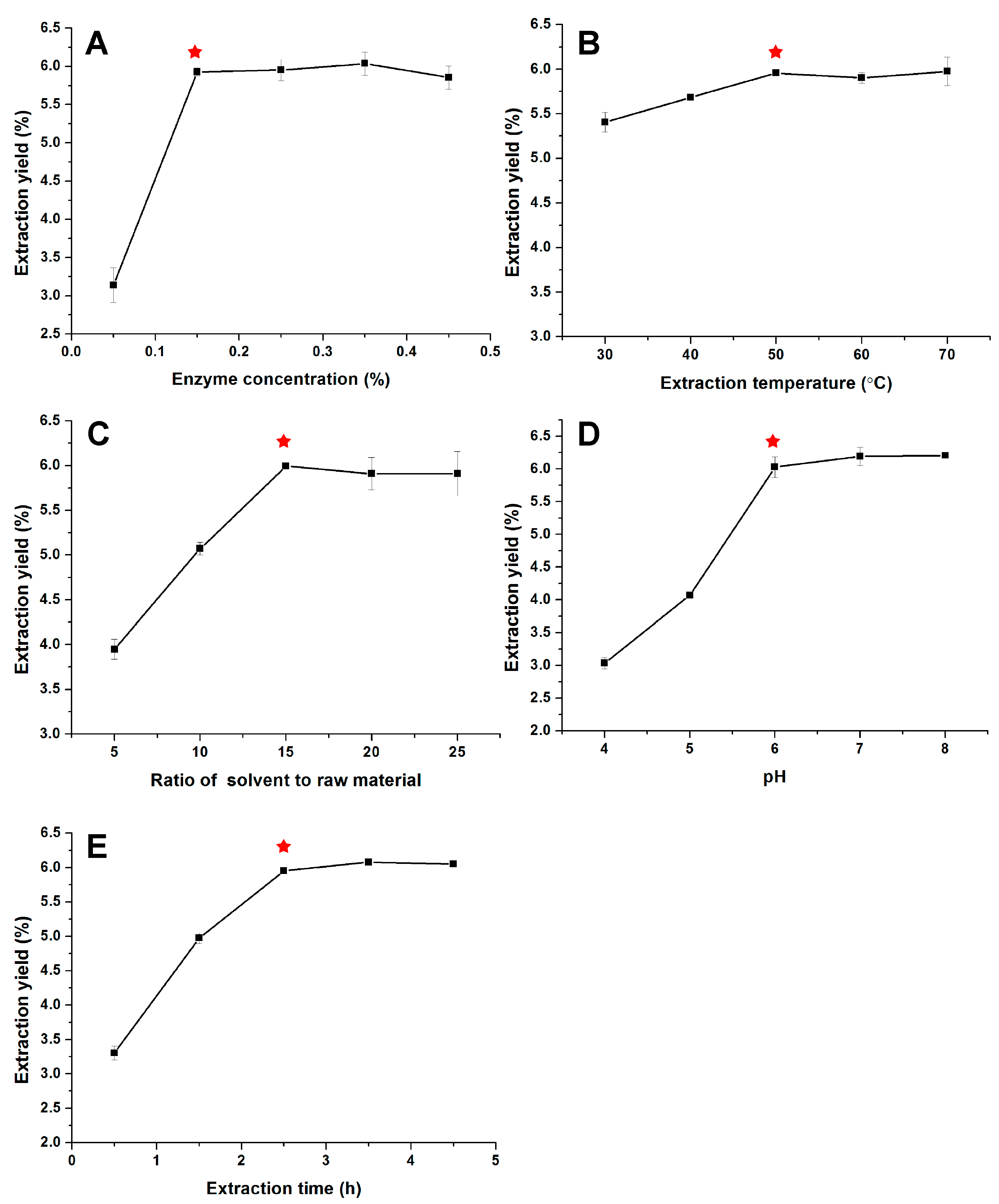
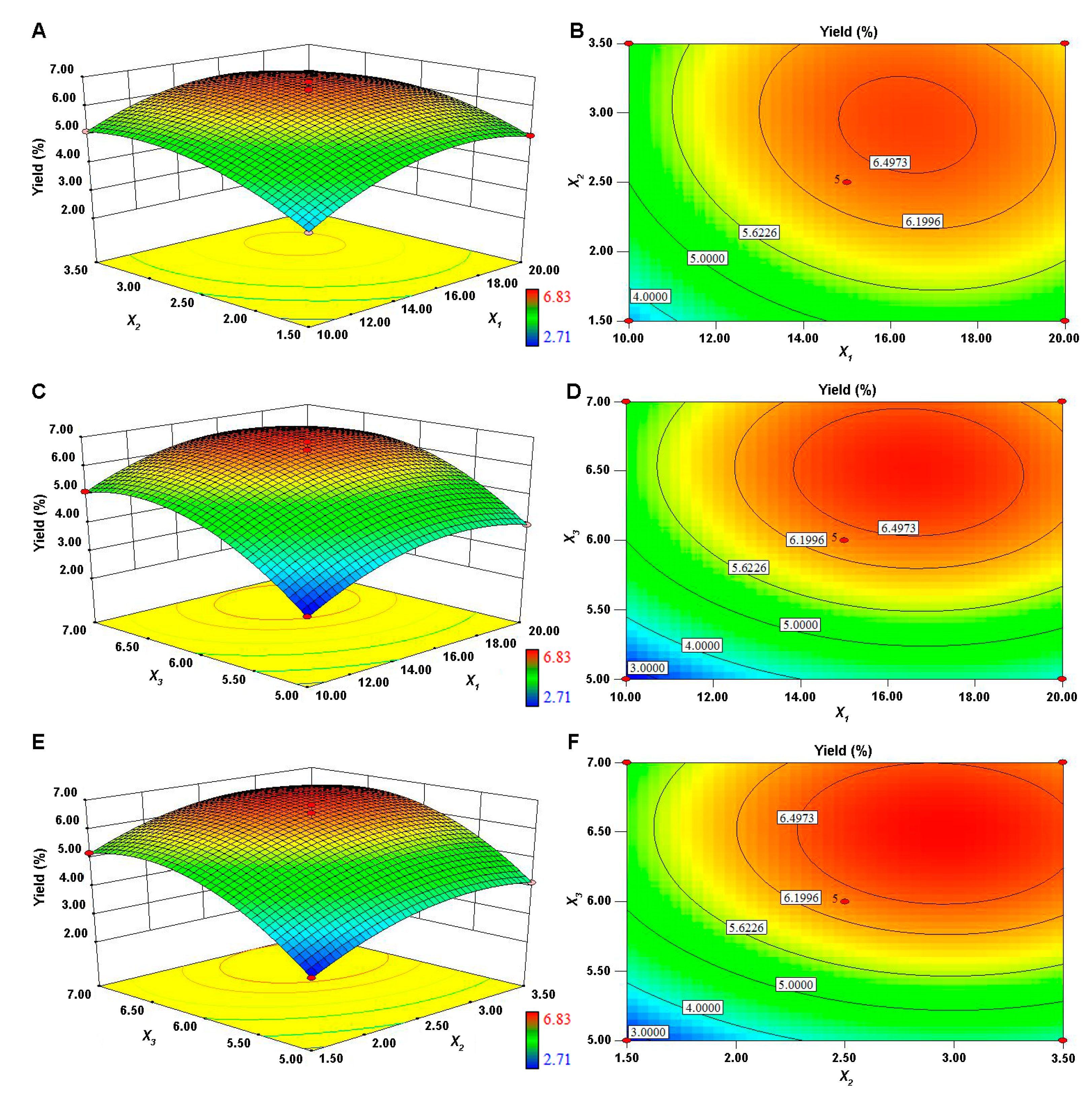
| Factors | Code | Levels and Range | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A: ratio of extraction solvent to raw material (mL/g) | X1 | 10 | 15 | 20 |
| B: extraction time (h) | X2 | 1.5 | 2.5 | 3.5 |
| C: extraction pH | X3 | 5 | 6 | 7 |
| Experiment | A: Ratio of Extraction Solvent to Raw Material (mL/g) | B: Extraction Time (h) | C: Extraction pH | Yield (%) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 3.48 |
| 2 | 1 | −1 | 0 | 4.97 |
| 3 | −1 | 1 | 0 | 5.12 |
| 4 | 1 | 1 | 0 | 5.86 |
| 5 | −1 | 0 | −1 | 2.71 |
| 6 | 1 | 0 | −1 | 3.95 |
| 7 | −1 | 0 | 1 | 5.12 |
| 8 | 1 | 0 | 1 | 5.97 |
| 9 | 0 | −1 | −1 | 2.78 |
| 10 | 0 | 1 | −1 | 4.15 |
| 11 | 0 | −1 | 1 | 5.18 |
| 12 | 0 | 1 | 1 | 6.24 |
| 13 | 0 | 0 | 0 | 6.83 |
| 14 | 0 | 0 | 0 | 6.03 |
| 15 | 0 | 0 | 0 | 6.32 |
| 16 | 0 | 0 | 0 | 6.56 |
| 17 | 0 | 0 | 0 | 6.14 |
| Source | Sum of Squares | df | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Model | 26.71 | 9 | 2.97 | 49.15 | <0.0001 ** |
| X1 | 2.33 | 1 | 2.33 | 38.63 | 0.0004 ** |
| X2 | 3.08 | 1 | 3.08 | 50.93 | 0.0002 ** |
| X3 | 9.95 | 1 | 9.95 | 164.72 | <0.0001 ** |
| X1X2 | 0.14 | 1 | 0.14 | 2.33 | 0.1708 |
| X1X3 | 0.038 | 1 | 0.038 | 0.63 | 0.4535 |
| X2X3 | 0.024 | 1 | 0.024 | 0.40 | 0.5482 |
| X12 | 2.93 | 1 | 2.93 | 48.53 | 0.0002 ** |
| X22 | 1.97 | 1 | 1.97 | 32.65 | 0.0007 ** |
| X32 | 5.13 | 1 | 5.13 | 85.03 | <0.0001 ** |
| Residual | 0.42 | 7 | 0.060 | ||
| Lack of fit | 0.00415 | 3 | 0.001383 | 0.013 | 0.9976 a |
| Pure error | 0.42 | 4 | 0.10 | ||
| Cor Total | 27.13 | 16 | |||
| R2 = 0.9844; Adj R2 = 0.9644; Pred R2 = 0.9735 | |||||
| Item | PPP | PPP-1a | PPP-1b | PPP-2 |
|---|---|---|---|---|
| Molecular weight (kDa) | / | 369.6 | 41.73 | 57.76 |
| Monosaccharide composition (mol) | ||||
| GlcA | 1.00 | ND a | ND | 1.00 |
| GalNAc | 2.34 | 1.00 | ND | 1.17 |
| Gal | 0.29 | 1.24 | ND | ND |
| Fuc | 9.41 | 8.04 | 1.00 | 1.77 |
| Protein content (%) | 3.62 | 0.14 | 0.01 | 0.02 |
| Sulfate group content (%) | 21.9 | 20.6 | 25.2 | 28.0 |
| SO3−/COO− | / | / | / | 3.32 |
| Intrinsic viscosity (mL/g) | 106.13 | 172.44 | 62.84 | 39.61 |
| Specific rotation | −83.22° | −77° | −138.5° | −42.5° |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Yuan, Q.; Zhang, Y.; Li, J.; Zhu, X.; Zhao, L.; Wen, J.; Liu, J.; Zhao, L.; Zhao, J. Enzyme-Assisted Extraction Optimization, Characterization and Antioxidant Activity of Polysaccharides from Sea Cucumber Phyllophorus proteus. Molecules 2018, 23, 590. https://doi.org/10.3390/molecules23030590
Qin Y, Yuan Q, Zhang Y, Li J, Zhu X, Zhao L, Wen J, Liu J, Zhao L, Zhao J. Enzyme-Assisted Extraction Optimization, Characterization and Antioxidant Activity of Polysaccharides from Sea Cucumber Phyllophorus proteus. Molecules. 2018; 23(3):590. https://doi.org/10.3390/molecules23030590
Chicago/Turabian StyleQin, Yujing, Qingxia Yuan, Yuexing Zhang, Jialu Li, Xinjiao Zhu, Lingling Zhao, Jing Wen, Jikai Liu, Longyan Zhao, and Jinhua Zhao. 2018. "Enzyme-Assisted Extraction Optimization, Characterization and Antioxidant Activity of Polysaccharides from Sea Cucumber Phyllophorus proteus" Molecules 23, no. 3: 590. https://doi.org/10.3390/molecules23030590





