Forsythoside A Modulates Zymosan-Induced Peritonitis in Mice
Abstract
:1. Introduction
2. Results
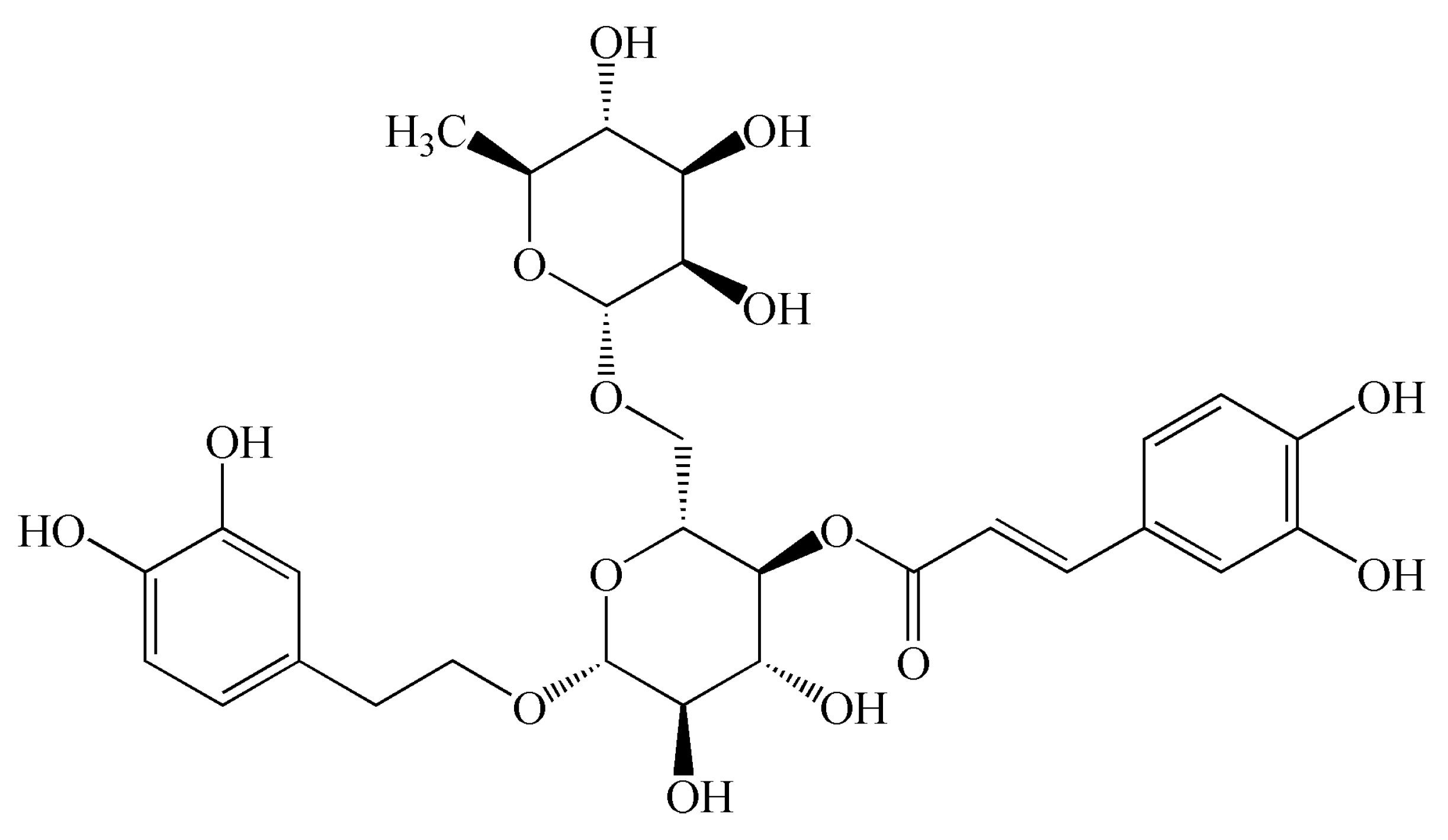
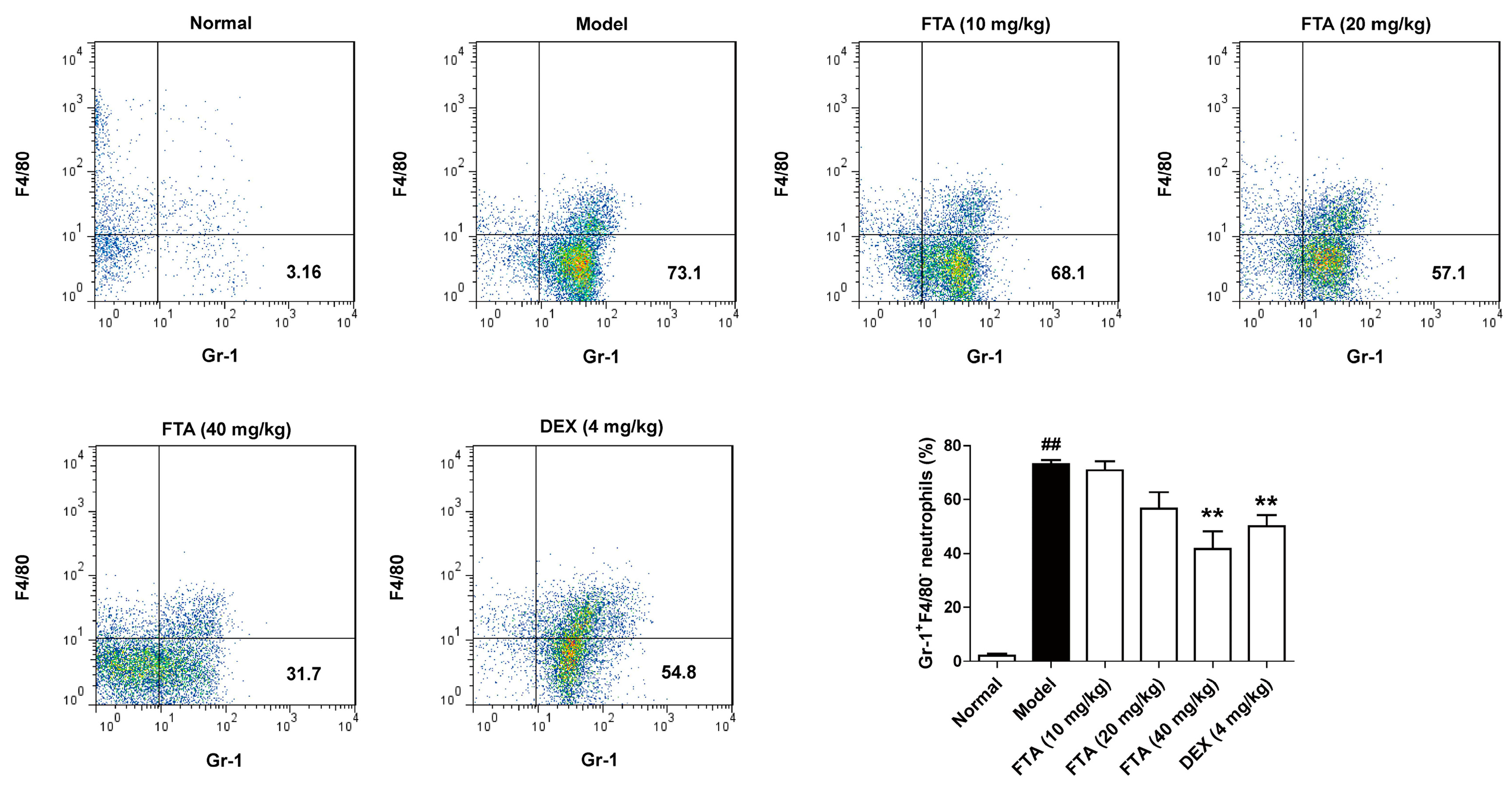
2.1. Treatment with FTA Decreases Neutrophils Numbers in the Peritoneal Cavity after Induction of Peritonitis
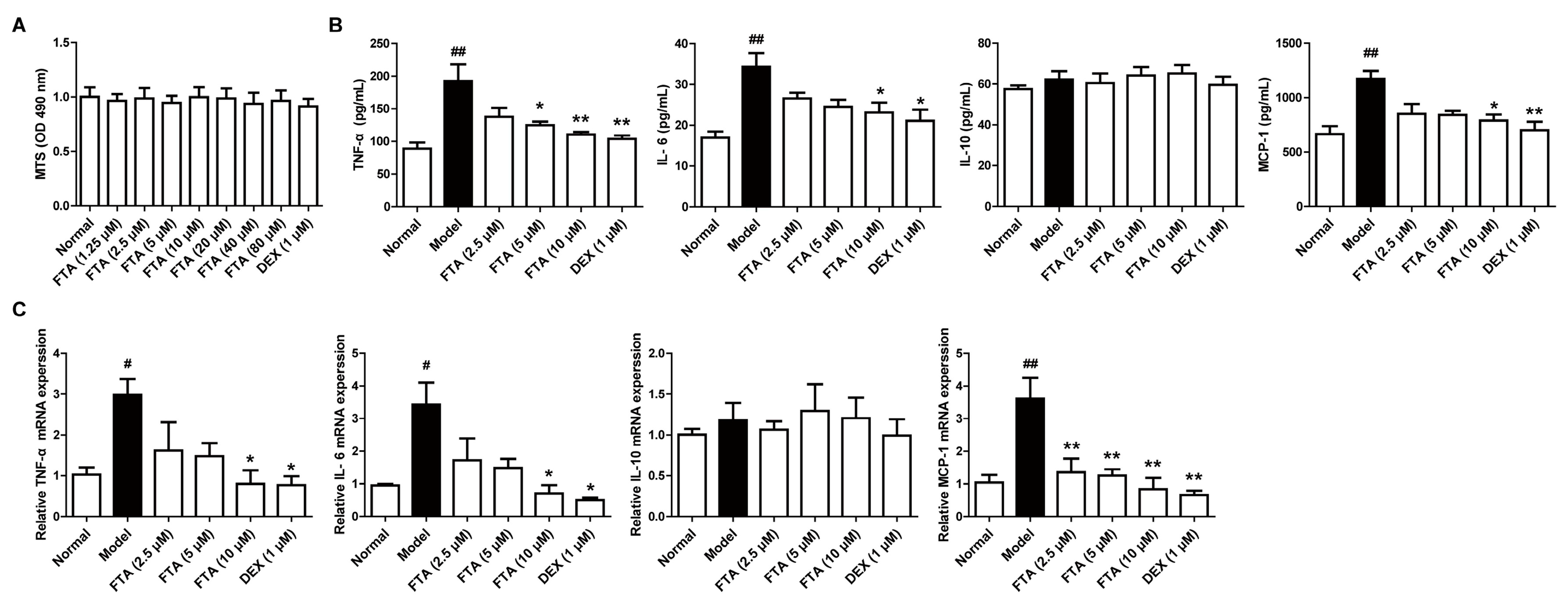
2.2. FTA Treatment Influences Cytokines Levels in the Peritoneal Cavity
2.3. FTA Reduces the Expressions of Cytokines in Zymosan-Stimulated RAW 264.7 Cells
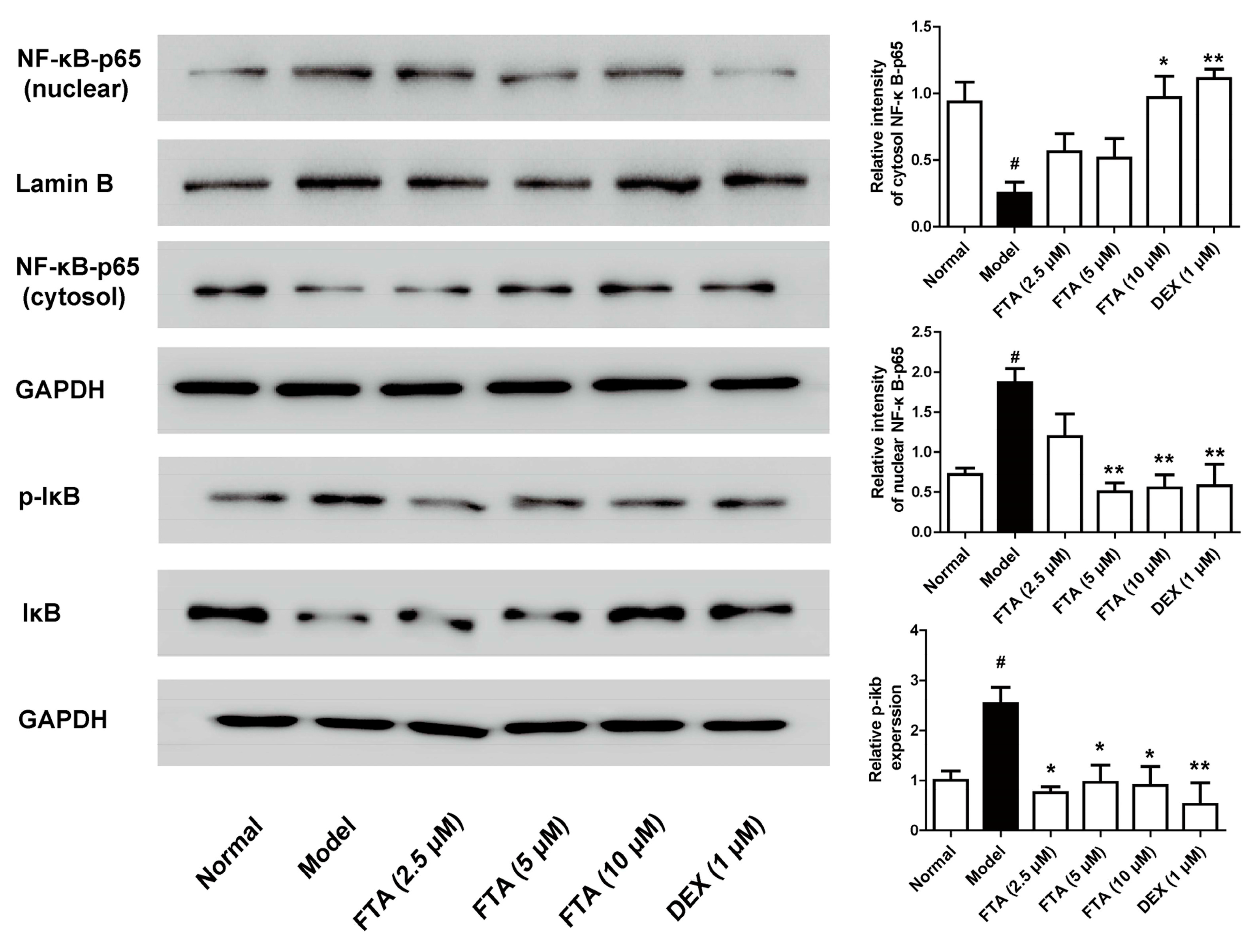
2.4. FTA Inhibits Zymosan-Induced Inflammatory Mediators through Activating NF-κB
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Zymosan-Induced Acute Peritonitis in Mice
4.4. Antibodies and Flow Cytometry Analysis
4.5. Cytokines and Chemokine Detection
4.6. Cell Culture and Treatment
4.7. MTS Assays
4.8. Quantitative Real-Time (QRT) PCR Assay
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newson, J.; Stables, M.; Karra, E.; Arce-Vargas, F.; Quezada, S.; Motwani, M.; Mack, M.; Yona, S.; Audzevich, T.; Gilroy, D.W. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood 2014, 124, 1748–1764. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock(sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Takatani, Y.; Higashi, T.; Inaba, M.; Ejima, T. Severe infection in critical emergency care. Nihon Rinsho 2016, 74, 245–251. [Google Scholar] [PubMed]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007, 21, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Xia, Q.; Liu, X.; Liu, W.; Huang, W.; Mei, X.; Luo, J.; Shan, M.; Lin, R.; Zou, D.; et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. J. Ethnopharmacol. 2018, 210, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, D.G.; Li, W.; Yang, H.J.; Yim, N.H.; Ma, J.Y. Anti-inflammatory effects of Forsythia suspense in dextran sulfate sodium-induced colitis. J. Ethnopharmacol. 2017, 206, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Hung, H.Y.; Nian, C.W.; Hwang, T.L.; Cheng, J.C.; Kuo, D.H.; Lee, E.J.; Tai, S.H.; Wu, T.S. Chemical Constituents and Anti-inflammatory Principles from the Fruits of Forsythia suspensa. J. Nat. Prod. 2017, 80, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Ding, R.B.; Liang, Y.; Liu, F.; Wang, K.; Jia, X.; Zhang, C.; Chen, M.; Li, P.; Su, H.; et al. Differences in Chemical Component and Anticancer Activity of Green and Ripe Forsythiae Fructus. Am. J. Chin. Med. 2017, 45, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Law, A.H.; Yang, C.L.; Lau, A.S.; Chan, G.C. Antiviral effect of forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit against influenza A virus through reduction of viral M1 protein. J. Ethnopharmacol. 2017, 209, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, J.; Zhang, Z.; Ma, Y.; Liao, F.; Zhang, Y.; Wu, G. Forsythoside a inhibits the avian infectious bronchitis virus in cell culture. Phytother. Res. 2011, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Zhang, Y.; Wang, Y.; Li, B.; Sun, W. Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspensa. J. Pharm. Pharmacol. 2008, 60, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Su, H.; Wan, H.; Qin, Q.; Wu, X.; Kong, X.; Lin, N. Forsythoside A exerts antipyretic effect on yeast-induced pyrexia mice via inhibiting transient receptor potential vanilloid 1 function. Int. J. Biol. Sci. 2017, 13, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jie, C.; Tang, L.P.; Meng, H.; Li, X.B.; Li, Y.B.; Chen, L.X.; Yan, C.; Kurihara, H.; Li, Y.F.; et al. New insights into the effects and mechanism of a classic traditional Chinese medicinal formula on influenza prevention. Phytomedicine 2017, 27, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Wang, L.W.; Liu, X.M.; Li, C.L.; Xu, S.P.; Farooq, A.D. Neuroprotective effects of forsythiaside on learning and memory deficits in senescence-accelerated mouse prone (SAMP8) mice. Pharmacol. Biochem. Behav. 2013, 105, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, S.; Kim, D.H.; Lee, C.H.; Park, S.J.; Jung, J.W.; Ko, K.H.; Cheong, J.H.; Lee, S.H.; Ryu, J.H. Neuroprotective effect of forsythiaside against transient cerebral global ischemia in gerbil. Eur. J. Pharmacol. 2011, 660, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.J.; Weng, X.G.; Cai, D.J.; Zhang, W.; Wang, J.F. Forsythoside A Inhibits BVDV Replication via TRAF2-Dependent CD28-4-1BB Signaling in Bovine PBMCs. PLoS ONE 2016, 11, e0162791. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.W.; Zhou, G.Y.; Chen, W.L.; Zhuge, L.; Jin, L.X.; Zheng, Y.; Lin, W.; Pan, Z.Z. Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. Int. Immunopharmacol. 2015, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ma, X.; Xun, Y.; Pan, L. Protective effect of forsythiaside A on OVA-induced asthma in mice. Eur. J. Pharmacol. 2017, 812, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Alves, A.K.; Galvão, J.G.; Teixeira, M.P.; Cavalcante-Silva, L.H.; Scavone, C.; Morrot, A.; Rumjanek, V.M.; Rodrigues-Mascarenhas, S. Ouabain Modulates Zymosan-Induced Peritonitis in Mice. Mediat. Inflamm. 2015, 2015, 265798. [Google Scholar] [CrossRef] [PubMed]
- McClure, C.; Brudecki, L.; Yao, Z.Q.; McCall, C.E.; El Gazzar, M. Processing Body Formation Limits Proinflammatory Cytokine Synthesis in Endotoxin-Tolerant Monocytes and Murine Septic Macrophages. J. Innate Immun. 2015, 7, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Cai, J.; Peng, Y.; Xiao, H.; Zhang, L.; Chen, J.; Liu, H. Protective effect of a novel antibody against TLR2 on zymosan-induced acute peritonitis in NF-κB transgenic mice. Am. J. Transl. Res. 2017, 9, 692–699. [Google Scholar] [PubMed]
- Zhang, J.; Zhang, Y.; Huang, H.; Zhang, H.; Lu, W.; Fu, G.; Zhu, Y. Forsythoside A inhibited S. aureus stimulated inflammatory response in primary bovine mammary epithelial cells. Microb. Pathog. 2018, 116, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Bai, X.Y.; Liu, Q.B.; Liu, S.; Gao, P.Y.; Li, L.Z.; Song, S.J. Two new glycosides from the fruits of Forsythia suspense. J. Asian Nat. Prod. Res. 2014, 16, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Y.; Yuan, W.; Zhou, L.; Wang, S.X.; Xie, Y.; Fu, Y.J. Forsythoside A exerts an anti-endotoxin effect by blocking the LPS/TLR4 signaling pathway and inhibiting Tregs in vitro. Int. J. Mol. Med. 2017, 40, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, Y.; Su, H.; Ye, D. Forsythiaside protects against hydrogen peroxide-induced oxidative stress and apoptosis in PC12 cell. Neurochem. Res. 2015, 40, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.I.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Pang, Q.; Song, S.; Miao, R.; Chen, W.; Zhou, Y.; Liu, C. The protective role of curcumin in zymosan-induced multiple organ dysfunction syndrome in mice. Shock 2016, 45, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, S.; Ono, S.; Tsujimoto, H.; Kinoshita, M.; Takahata, R.; Miyazaki, H.; Saitoh, D.; Hase, K. Neutralization of interleukin-10 or transforming growth factor-β decreases the percentages of CD4+ CD25+ Foxp3+ regulatory T cells in septic mice, thereby leading to an improved survival. Surgery 2012, 151, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Schotte, H.; Schlüter, B.; Schmidt, H.; Gaubitz, M.; Drynda, S.; Kekow, J.; Willeke, P. Putative IL-10 Low Producer Genotypes Are Associated with a Favourable Etanercept Response in Patients with Rheumatoid Arthritis. PLoS ONE 2015, 10, e0130907. [Google Scholar] [CrossRef] [PubMed]
- Watzlawick, R.; Kenngott, E.E.; Liu, F.D.; Schwab, J.M.; Hamann, A. Anti-Inflammatory Effects of IL-27 in Zymosan-Induced Peritonitis: Inhibition of Neutrophil Recruitment Partially Explained by Impaired Mobilization from Bone Marrow and Reduced Chemokine Levels. PLoS ONE 2015, 10, e0137651. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Takahashi, M. IFN-γ-mediated survival enables human neutrophils to produce MCP-1/CCL2 in response to activation by TLR ligands. J. Immunol. 2007, 179, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, L.; Tian, L.; Li, W.; Yang, L.; Li, L. Macrophage Migration Inhibitor Factor Upregulates MCP-1 Expression in an Autocrine Manner in Hepatocytes during Acute Mouse Liver Injury. Sci. Rep. 2016, 6, 27665. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Sorting the signals from the signals in the noisy environment of inflammation. Sci. Signal. 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Lluch, J.; Almudever, P.; Freire, J.; Xiaozhong, Q.; Cortijo, J. Roflumilast N-oxide reverses corticosteroid resistance in neutrophils from patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2014, 134, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Lin, C.; Ren, J.; Zhang, S. Forsythiaside A exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia cells through activation of Nrf2/HO-1 signaling pathway. Neurochem. Res. 2016, 41, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhao, Y.; Li, H.; Wu, Y.; Li, X.; Han, Q.; Dai, C.; Li, Y. Forsythiaside attenuates lipopolysaccharide-induced inflammatory responses in the bursa of Fabricius of chickens by downregulating the NF-κB signaling pathway. Exp. Ther. Med. 2014, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, F.; Ma, R.; Hu, X. Forsythiaside inhibits cigarette smoke-induced lung inflammation by activation of Nrf2 and inhibition of NF-κB. Int. Immunopharmacol. 2015, 28, 494–499. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-T.; Ding, Y.; Kang, P.; Zhang, X.-Y.; Zhang, T. Forsythoside A Modulates Zymosan-Induced Peritonitis in Mice. Molecules 2018, 23, 593. https://doi.org/10.3390/molecules23030593
Zhang X-T, Ding Y, Kang P, Zhang X-Y, Zhang T. Forsythoside A Modulates Zymosan-Induced Peritonitis in Mice. Molecules. 2018; 23(3):593. https://doi.org/10.3390/molecules23030593
Chicago/Turabian StyleZhang, Xiao-Tian, Yue Ding, Ping Kang, Xin-Yu Zhang, and Tong Zhang. 2018. "Forsythoside A Modulates Zymosan-Induced Peritonitis in Mice" Molecules 23, no. 3: 593. https://doi.org/10.3390/molecules23030593



