Cyclization Reaction of Acyl Thiourea Chitosan: Enhanced Antifungal Properties via Structural Optimization
Abstract
:1. Introduction
2. Results and Discussion
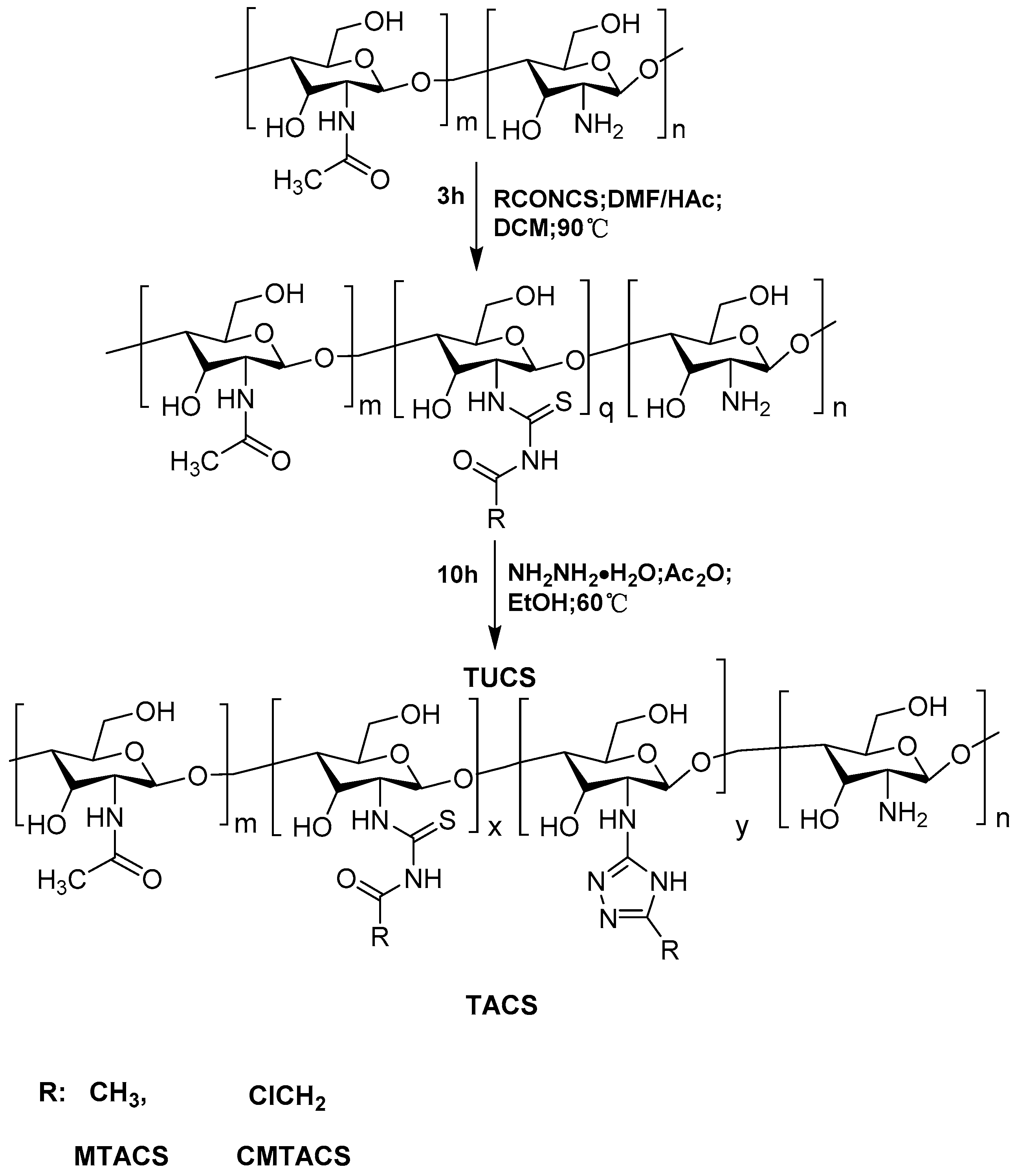
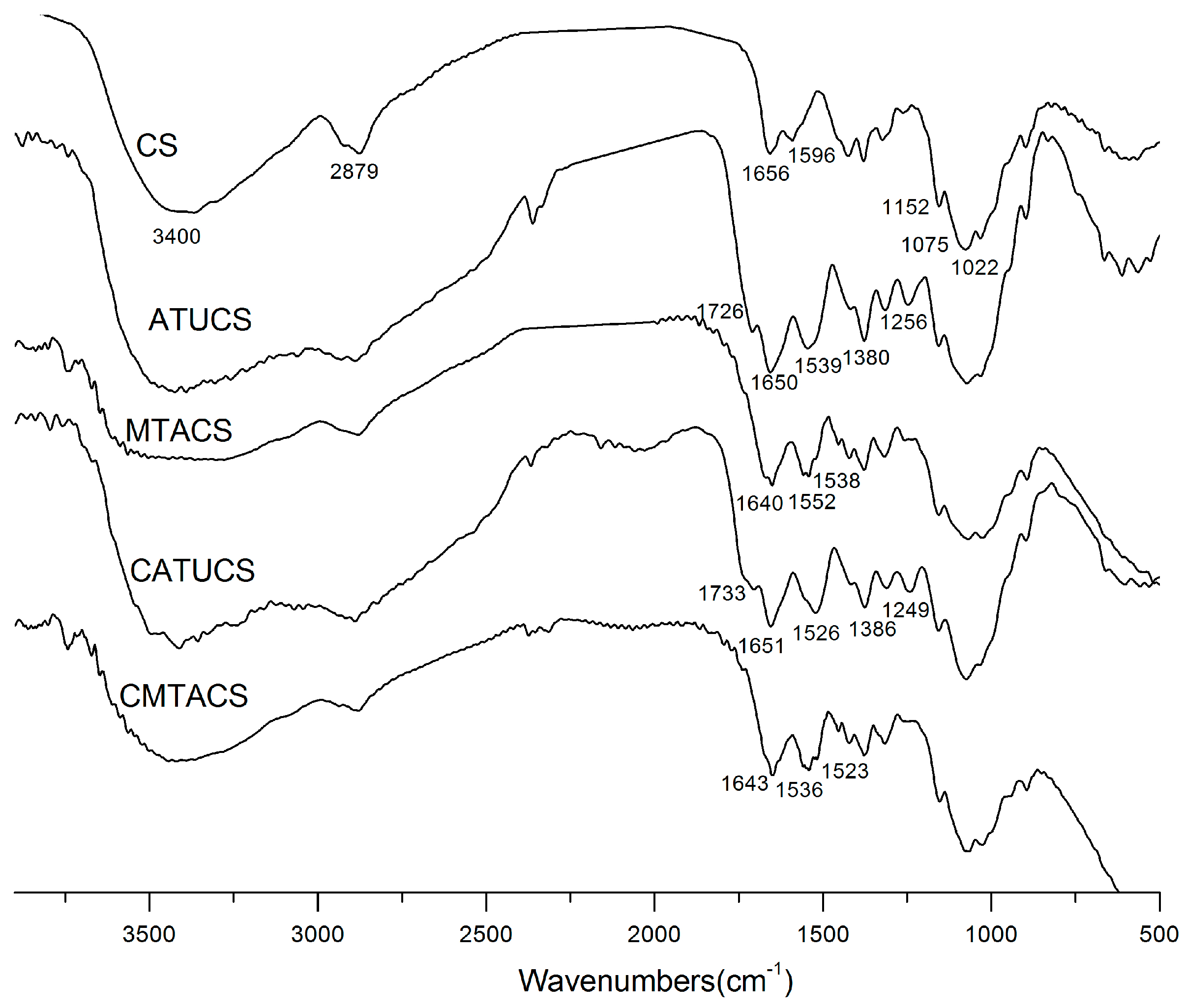
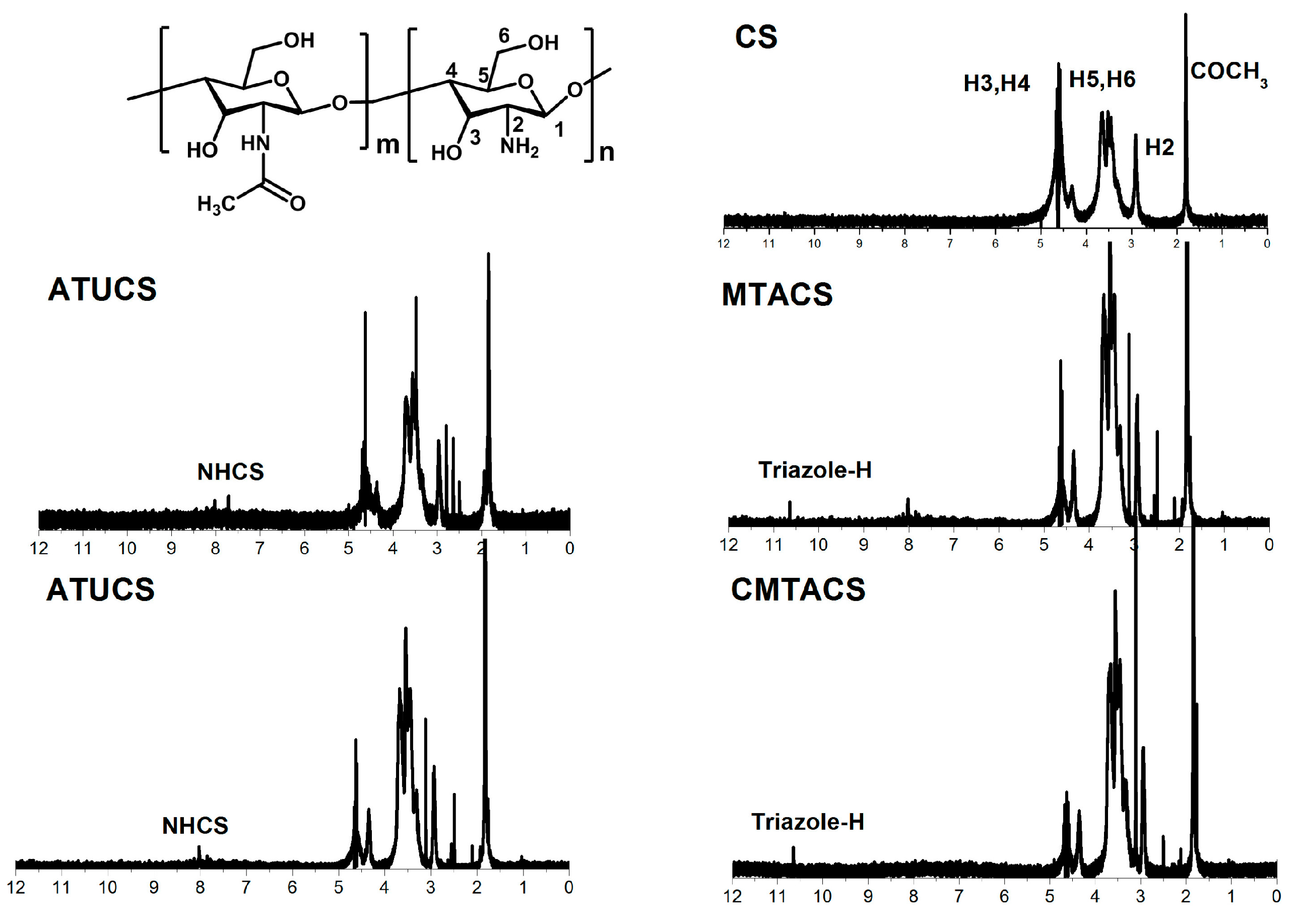
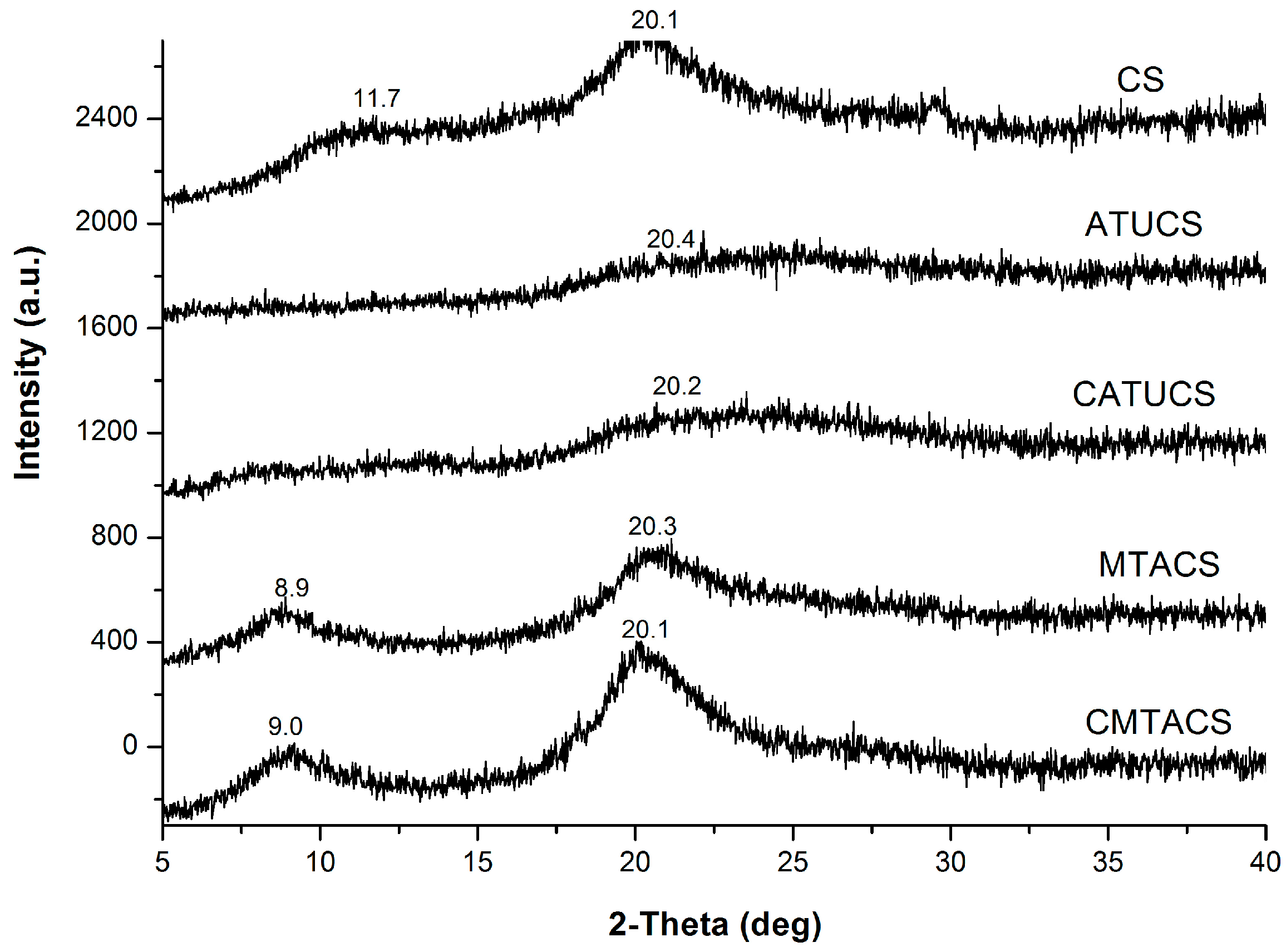
2.1. Preparation and Characterization of 3-Methyl-1,2,4-triazolyl Chitosan (MTACS) and 3-Chloromethyl-1,2,4-triazolyl Chitosan (CMTACS) Derivatives
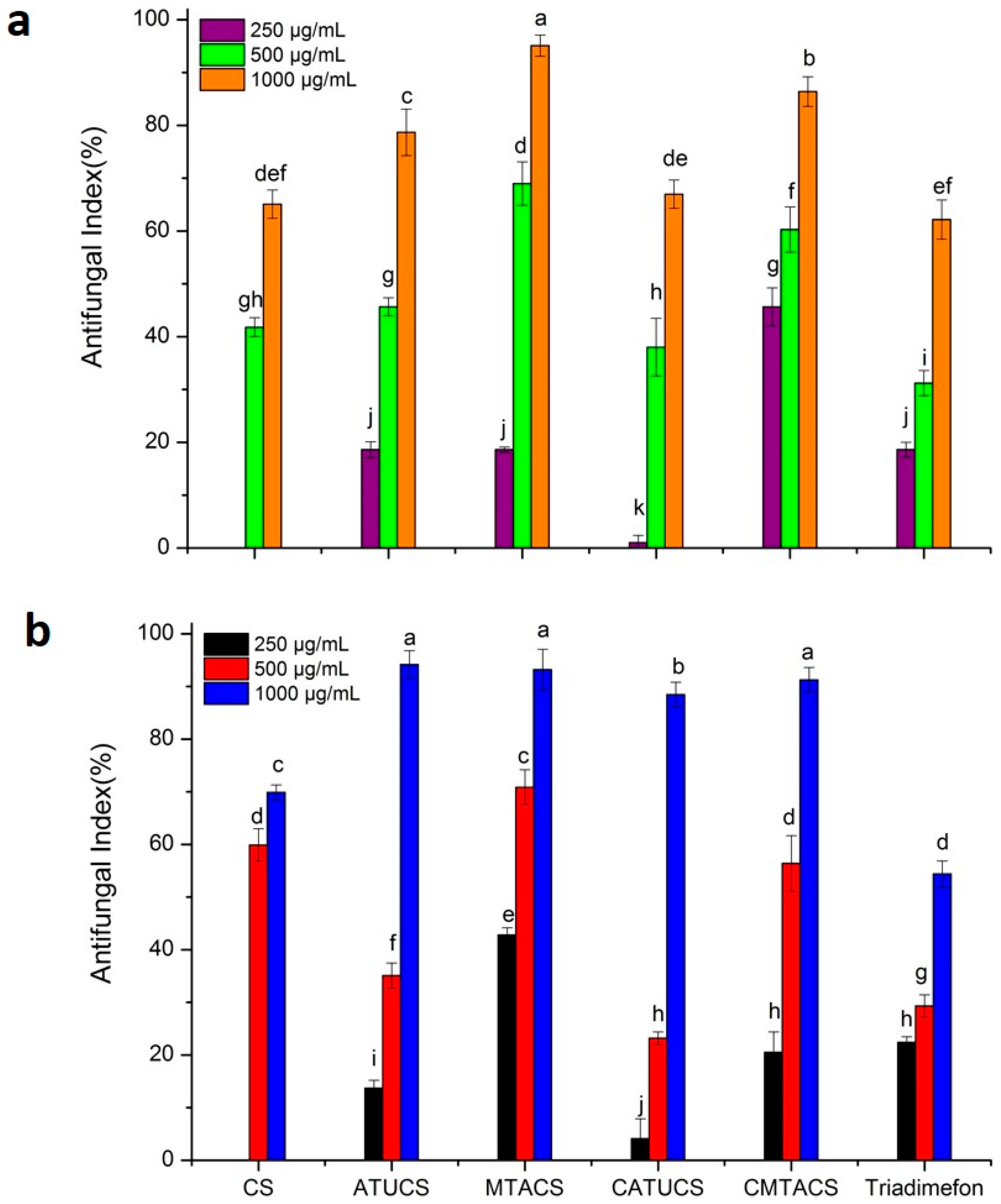
2.2. Antifungal Activity of 3-Methyl-1,2,4-triazolyl Chitosan (MTACS) Derivative
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Synthesis of 3-Methyl-1,2,4-triazolyl Chitosan (MTACS) Derivative and 3-Chloromethyl-1,2,4-triazolyl Chitosan (CMTACS) Derivative
3.4. Antifungal Activity in Vitro
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3-methyl-1,2,4-triazolyl chitosan | MTACS |
| 3-chloromethyl-1,2,4-triazolyl chitosan | CMTACS |
| Acetyl thiourea chitosan | ATUCS |
| Chloroacetyl thiourea chitosan | CATUCS |
| Chitosan | CS |
| Stemphylium solani weber | S. solani |
| Alternaria porri | A. porri |
| Gloeosporium theae-sinensis | G. theae-sinensis |
| Fourier transform infrared | FTIR |
| Nuclear magnetic resonance | NMR |
| X-ray diffraction | XRD |
| Differential scanning calorimetry | DSC |
| Scanning electron microscopy | SEM |
| Potato dextrose agar | PDA |
| N,N-Dimethylformamide | DMF |
References
- Hollomon, D.W.; Brent, K.J. Combating plant diseases—The Darwin connection. Pest Manag. Sci. 2009, 65, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.M.; El-Shehedi, A. The mode of penetration of Alternaria porri into onion leaves. Trans. Br. Mycol. Soc. 1966, 49, 79–80. [Google Scholar] [CrossRef]
- Chai, A.L.; Du, G.-F.; Shi, Y.-X.; Xie, X.-W.; Li, B.-J. Leaf Spot on Sweet Potato (Ipomoea batatas) Caused by Stemphylium solani, a New Disease in China. J. Phytopathol. 2015, 163, 1046–1049. [Google Scholar] [CrossRef]
- Zheng, L.; Lv, R.; Hsiang, T.; Huang, J. Host range and phytotoxicity of Stemphylium solani, causing leaf blight of garlic (Allium sativum) in China. Eur. J. Plant Pathol. 2009, 124, 21–30. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, W.; Zeng, L.; Shen, D.; Liu, Z.; Wang, X.; Tong, H. Characterization, Pathogenicity, and Phylogenetic Analyses of Colletotrichum Species Associated with Brown Blight Disease on Camellia sinensis in China. Plant Dis. 2017, 101, 1022–1028. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Collison, E.; Hird, H.; Cresswell, J.; Tyler, C. Interactive effects of pesticide exposure and pathogen infection on bee health—a critical analysis. Biol. Rev. 2016, 91, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Mohimont, L.; Court-Marques, D.; Istace, F.; Jacobs, M.; Sebestyen, I.; Steinkellner, H.; Tiramani, M. Pesticide risk assessment at the European Food Safety Authority: New developments. Toxicol. Lett. 2010, 196, S25. [Google Scholar] [CrossRef]
- Hou, B.; Wu, L. Safety impact and farmer awareness of pesticide residues. Food Agric. Immunol. 2010, 21, 191–200. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Periayah, M.; Halim, A.; Saad, A. Chitosan: A promising marine polysaccharide for biomedical research. Pharmacogn. Rev. 2016, 10, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Galván Márquez, I.; Akuaku, J.; Cruz, I.; Cheetham, J.; Golshani, A.; Smith, M.L. Disruption of protein synthesis as antifungal mode of action by chitosan. Int. J. Food Microbiol. 2013, 164, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Yu, H.; Zain-ul, A.; Chen, Y.; Chen, Q.; Zhou, W.; Zhang, H.; Chen, X. Recent progress on synthesis, property and application of modified chitosan: An overview. Int. J. Biol. Macromol. 2016, 88, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Saesoo, S.; Ovatlarnporn, C. Antifungal property of quaternized chitosan and its derivatives. Int. J. Biol. Macromol. 2012, 50, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, Z.; Jiang, P. Synthesis, characterization, and antifungal activity of novel quaternary chitosan derivatives. Carbohydr. Res. 2010, 345, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens. Carbohydr. Polym. 2014, 111, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Rabea, E.I. Synthesis and antifungal property of N-(aryl) and quaternary N-(aryl) chitosan derivatives against Botrytis cinerea. Cellulose 2014, 21, 3121–3137. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Synthesis and structure–activity relationship of N-(cinnamyl) chitosan analogs as antimicrobial agents. Int. J. Biol. Macromol. 2013, 57, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, S.; Xing, R.; Li, K.; Yu, H.; Li, P. Synthesis and antifungal evaluation of (1,2,3-triazol-4-yl)methyl nicotinate chitosan. Int. J. Biol. Macromol. 2013, 61, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis and antimicrobial activity of some novel terephthaloyl thiourea cross-linked carboxymethyl chitosan hydrogels. Cellulose 2012, 19, 1879–1891. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Mohamed, R.R.; Seoudi, R.S. Synthesis and characterization of some novel antimicrobial thiosemicarbazone O-carboxymethyl chitosan derivatives. Int. J. Biol. Macromol. 2014, 63, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xing, R.; Liu, S.; Yu, H.; Li, K.; Hu, L.; Li, P. Synthesis and antifungal properties of (4-tolyloxy)-pyrimidyl-α-aminophosphonates chitosan derivatives. Int. J. Biol. Macromol. 2014, 63, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, G.; Zeng, H. Preparation of high antimicrobial activity thiourea chitosan–Ag+ complex. Carbohydr. Polym. 2005, 60, 33–38. [Google Scholar] [CrossRef]
- Wang, L.; Xing, R.; Liu, S.; Yu, H.; Qin, Y.; Li, K.; Feng, J.; Li, R.; Li, P. Recovery of silver (I) using a thiourea-modified chitosan resin. J. Hazard. Mater. 2010, 180, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abd El-Ghany, N.A. Preparation and antimicrobial activity of some carboxymethyl chitosan acyl thiourea derivatives. Int. J. Biol. Macromol. 2012, 50, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, S.S.; Salem, H.A.; Eweis, M.; Elsabee, M.Z. Synthesis and characterization of some acyl thiourea derivatives of chitosan and their biocidal activities. Int. J. Biol. Macromol. 2014, 70, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohydr. Res. 2008, 343, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Palaska, E.; Şahin, G.; Kelicen, P.; Durlu, N.T.; Altinok, G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Il Farm. 2002, 57, 101–107. [Google Scholar] [CrossRef]
- Klix, M.B.; Verreet, J.-A.; Beyer, M. Comparison of the declining triazole sensitivity of Gibberella zeae and increased sensitivity achieved by advances in triazole fungicide development. Crop Prot. 2007, 26, 683–690. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Synthesis and characterization of a novel amphiphilic chitosan–polylactide graft copolymer. Carbohydr. Polym. 2005, 59, 165–171. [Google Scholar] [CrossRef]
- Elizalde-Peña, E.A.; Flores-Ramirez, N.; Luna-Barcenas, G.; Vásquez-García, S.R.; Arámbula-Villa, G.; García-Gaitán, B.; Rutiaga-Quiñones, J.G.; González-Hernández, J. Synthesis and characterization of chitosan-g-glycidyl methacrylate with methyl methacrylate. Eur. Polym. J. 2007, 43, 3963–3969. [Google Scholar] [CrossRef]
- Phillips, G.O.; Takigami, S.; Takigami, M. Hydration characteristics of the gum exudate from Acacia senegal. Food Hydrocoll. 1996, 10, 11–19. [Google Scholar] [CrossRef]
- Izabella Agostinho Pena, N.; Leticia, N.; Fabio Rogerio de, M.; Jose Augusto, S.; Fabian, V.-R.; Luiz, B.; Inacio Henrique, Y.; Ivan, M.; Ljubica, T.; Jose Gilberto, J.; et al. Computational Biology Tools for Identifying Specific Ligand Binding Residues for Novel Agrochemical and Drug Design. Curr. Protein Pept. Sci. 2015, 16, 701–717. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Elemental Analysis (%) | DSthiourea (%) | DStriazole (%) | |||
|---|---|---|---|---|---|---|
| C | N | H | S | |||
| CS | 40.05 | 7.29 | 6.41 | – | – | – |
| ATUCS | 39.66 | 8.67 | 6.73 | 4.11 | 33.7 | – |
| CATUCS | 38.30 | 7.81 | 6.80 | 3.01 | 27.0 | – |
| MTACS | 37.39 | 7.53 | 6.18 | 0.88 | 9.3 | 24.4 |
| CMTACS | 36.77 | 10.35 | 6.35 | 0.93 | 8.0 | 19.0 |
| Sample | Concentration (μg/mL) | A. porri | G. theae-sinensis | S. solani |
|---|---|---|---|---|
| Antifungal Index * | Antifungal Index * | Antifungal Index * | ||
| CS | 250 | 0 k | 0 j | 12.3 ± 1.3 i |
| 500 | 41.8 ± 1.8 g,h | 59.9 ±3.1 d | 41.2 ± 2.9 g | |
| 1000 | 65.1 ± 2.7 d,e,f | 69.9 ± 1.4 c | 50.9 ± 3.6 f | |
| ATUCS | 250 | 18.6 ± 1.5 j | 13.7 ± 1.5 i | 27.7 ± 1.2 h |
| 500 | 45.7 ± 1.7 g | 35.1 ± 2.4 f | 54.7 ± 2.7 e,f | |
| 1000 | 78.7 ± 4.4 c | 94.2 ± 2.6 a | 90.3 ± 3.0 b | |
| MTACS | 250 | 18.6 ± 0.5 j | 42.8 ± 1.4 e | 60.5 ± 2.5 e |
| 500 | 69.0 ± 4.1 d | 70.9 ± 3.3 c | 82.6 ± 4.2 c | |
| 1000 | 95.1± 2.0 a | 93.2 ± 3.9 a | 95.2 ± 3.7 a,b | |
| CATUCS | 250 | 1.0 ± 1.4 k | 4.1 ± 3.8 j | 26.8 ± 2.6 h |
| 500 | 38.0 ± 5.5 h | 23.2 ± 1.2 h | 54.7 ± 1.4 e,f | |
| 1000 | 67.0 ± 2.7 d,e | 88.5 ± 2.3 b | 70.1 ± 2.2 d | |
| CMTACS | 250 | 45.7 ± 3.6 g | 20.5 ± 3.9 h | 42.2 ± 6.3 g |
| 500 | 60.3 ± 4.3 f | 56.4 ± 5.3 d | 71.1 ± 2.4 d | |
| 1000 | 86.4 ± 2.8 b | 91.3 ± 2.3 a | 100 a | |
| Triadimefon | 250 | 18.6 ± 1.4 j | 22.4 ± 1.1 h | 100 a |
| 500 | 31.2 ± 2.4 i | 29.3 ± 2.1 g | 100 a | |
| 1000 | 62.2 ± 3.7 e,f | 54.4 ± 2.5 d | 100 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Liu, W.; Xing, R.; Liu, S.; Li, K.; Li, P. Cyclization Reaction of Acyl Thiourea Chitosan: Enhanced Antifungal Properties via Structural Optimization. Molecules 2018, 23, 594. https://doi.org/10.3390/molecules23030594
Qin Y, Liu W, Xing R, Liu S, Li K, Li P. Cyclization Reaction of Acyl Thiourea Chitosan: Enhanced Antifungal Properties via Structural Optimization. Molecules. 2018; 23(3):594. https://doi.org/10.3390/molecules23030594
Chicago/Turabian StyleQin, Yukun, Weixiang Liu, Ronge Xing, Song Liu, Kecheng Li, and Pengcheng Li. 2018. "Cyclization Reaction of Acyl Thiourea Chitosan: Enhanced Antifungal Properties via Structural Optimization" Molecules 23, no. 3: 594. https://doi.org/10.3390/molecules23030594





