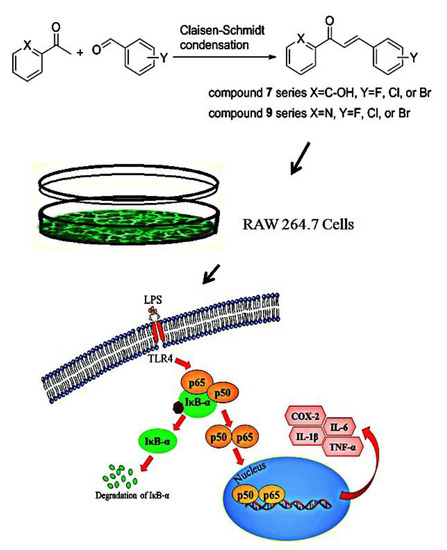
Halo-Substituted Chalcones and Azachalcones Inhibited Lipopolysaccharited-Stimulated Pro-Inflammatory Responses through the TLR4-Mediated Pathway
Abstract
:1. Introduction
2. Results and Discussion
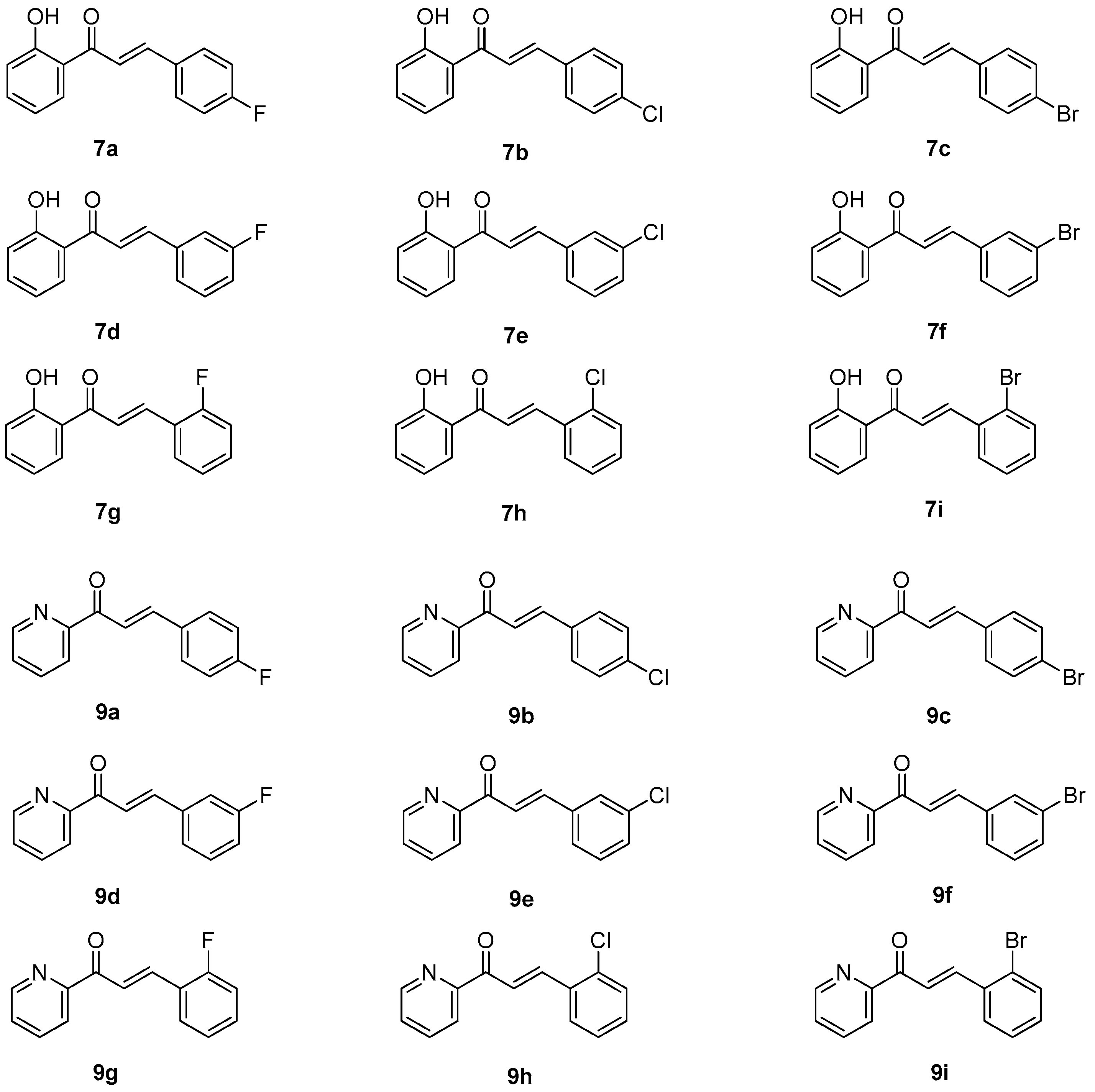
2.1. Synthesis of Halo-Substituted Chalcones 7a–i and Azachalcones 9a–i
2.2. Biological Evaluation
2.2.1. Cytotoxic Effect of Halo-Substituted Chalcones (7a–i) and Azachalcones (9a–i)
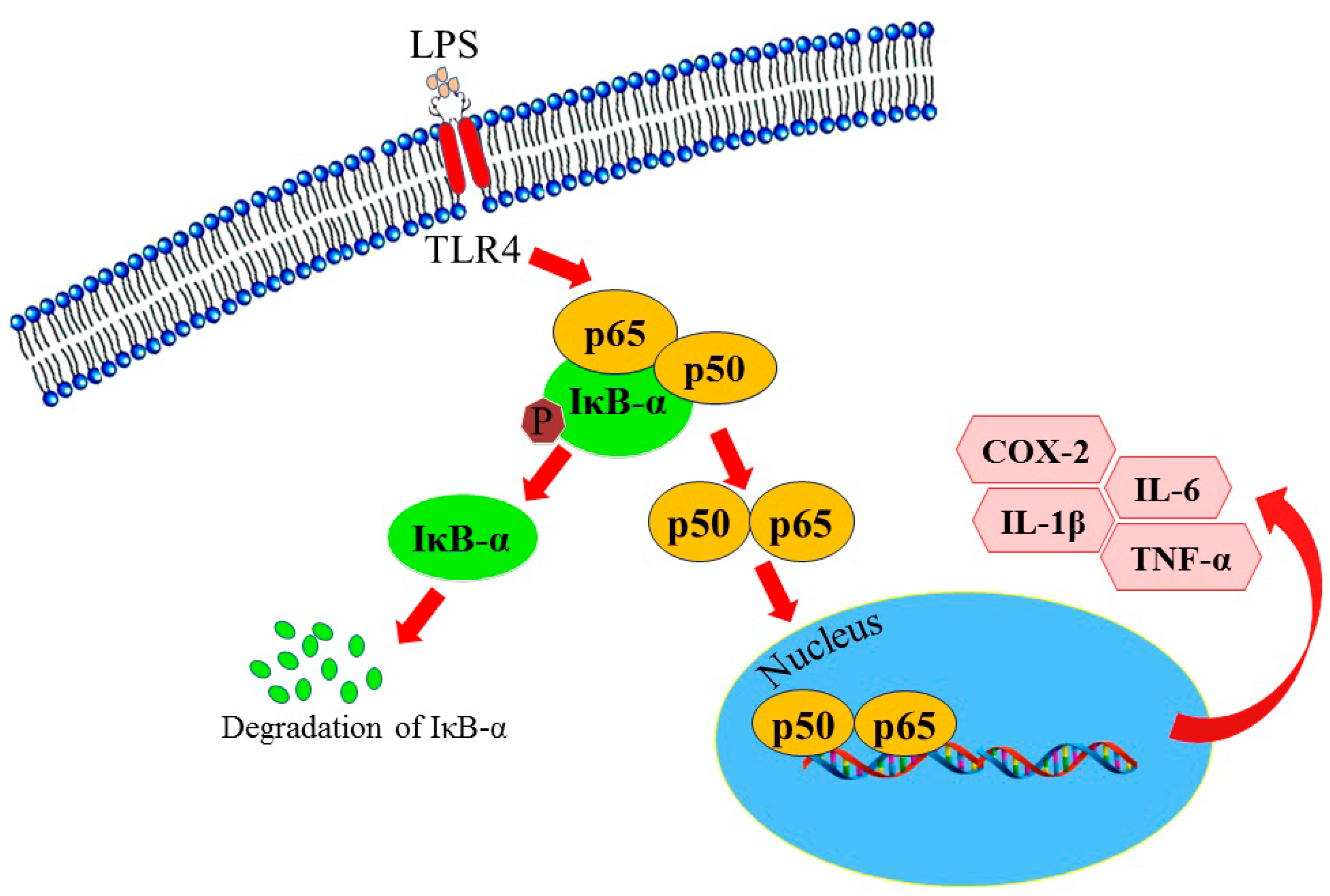
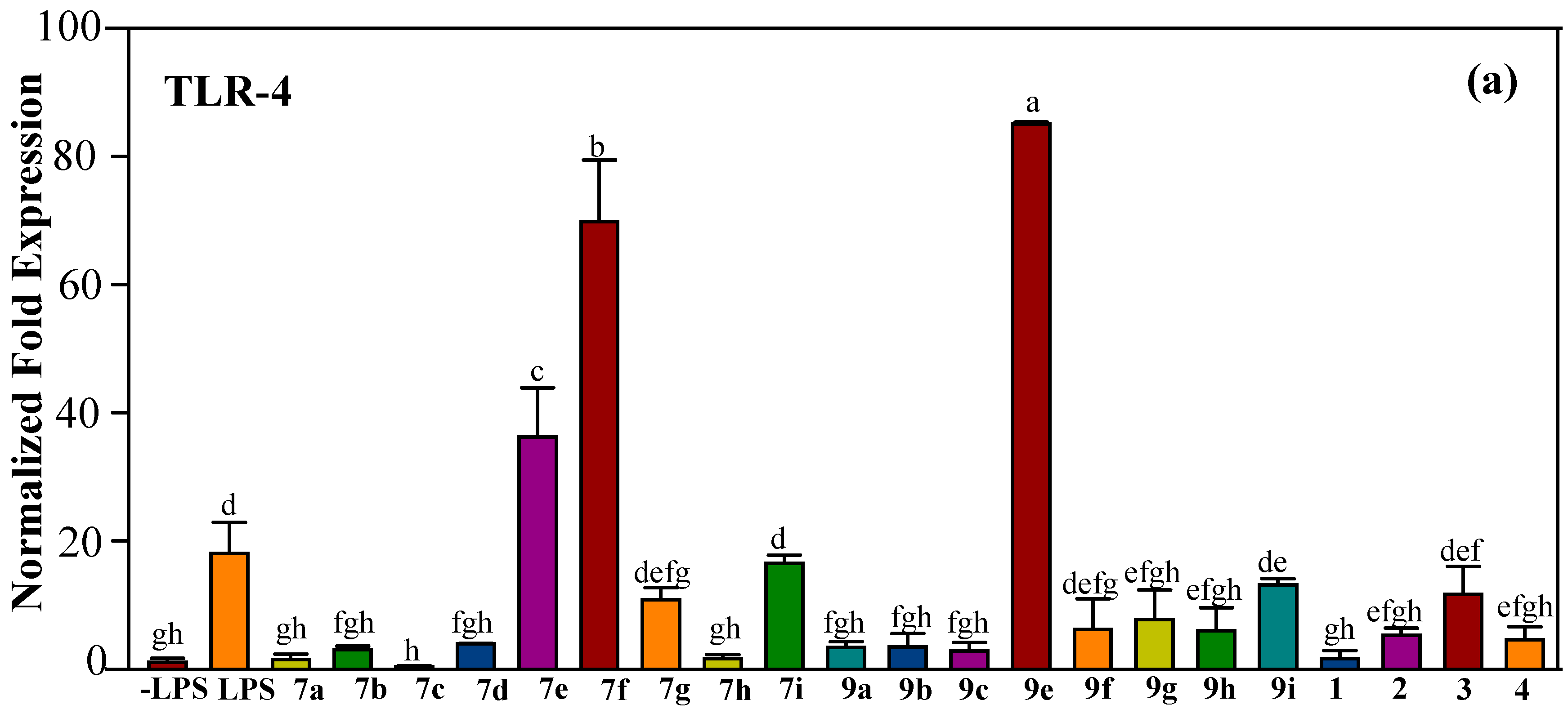
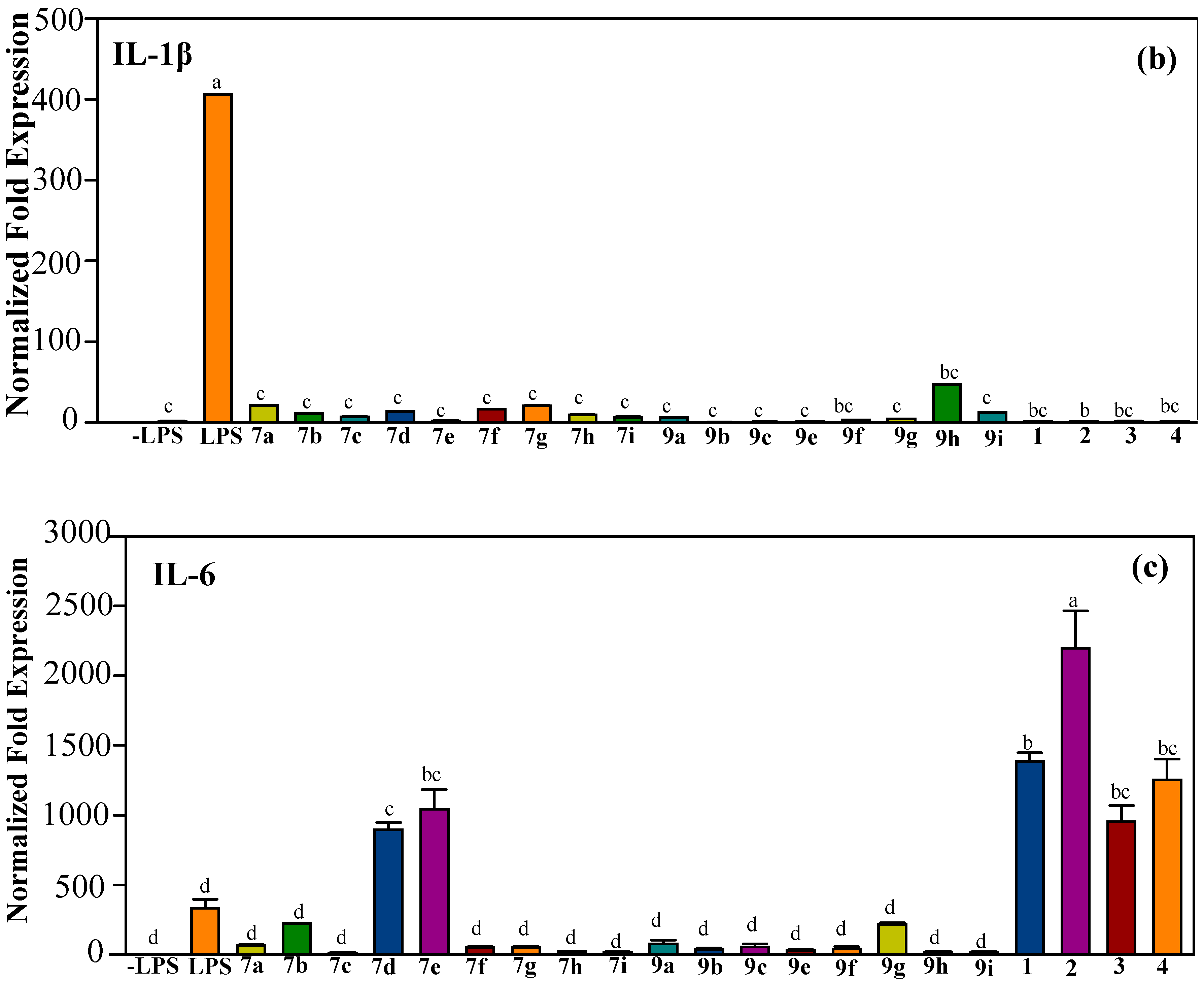
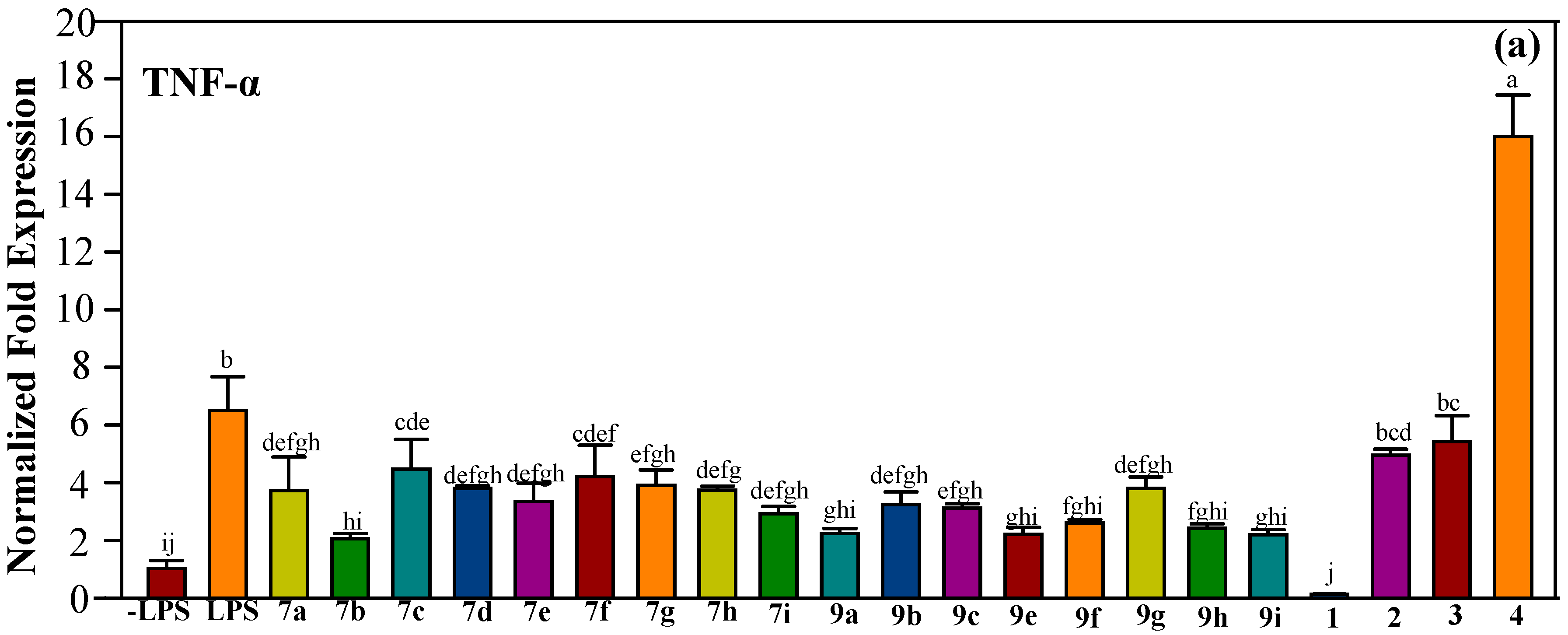
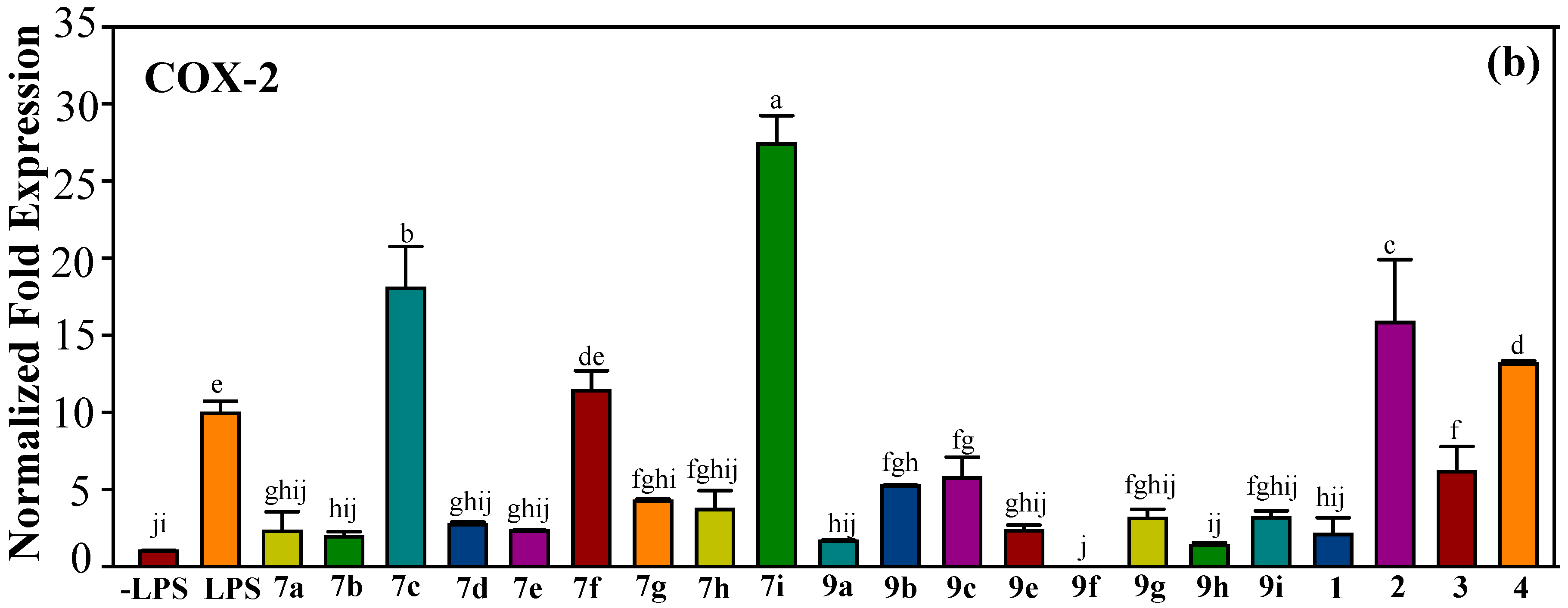
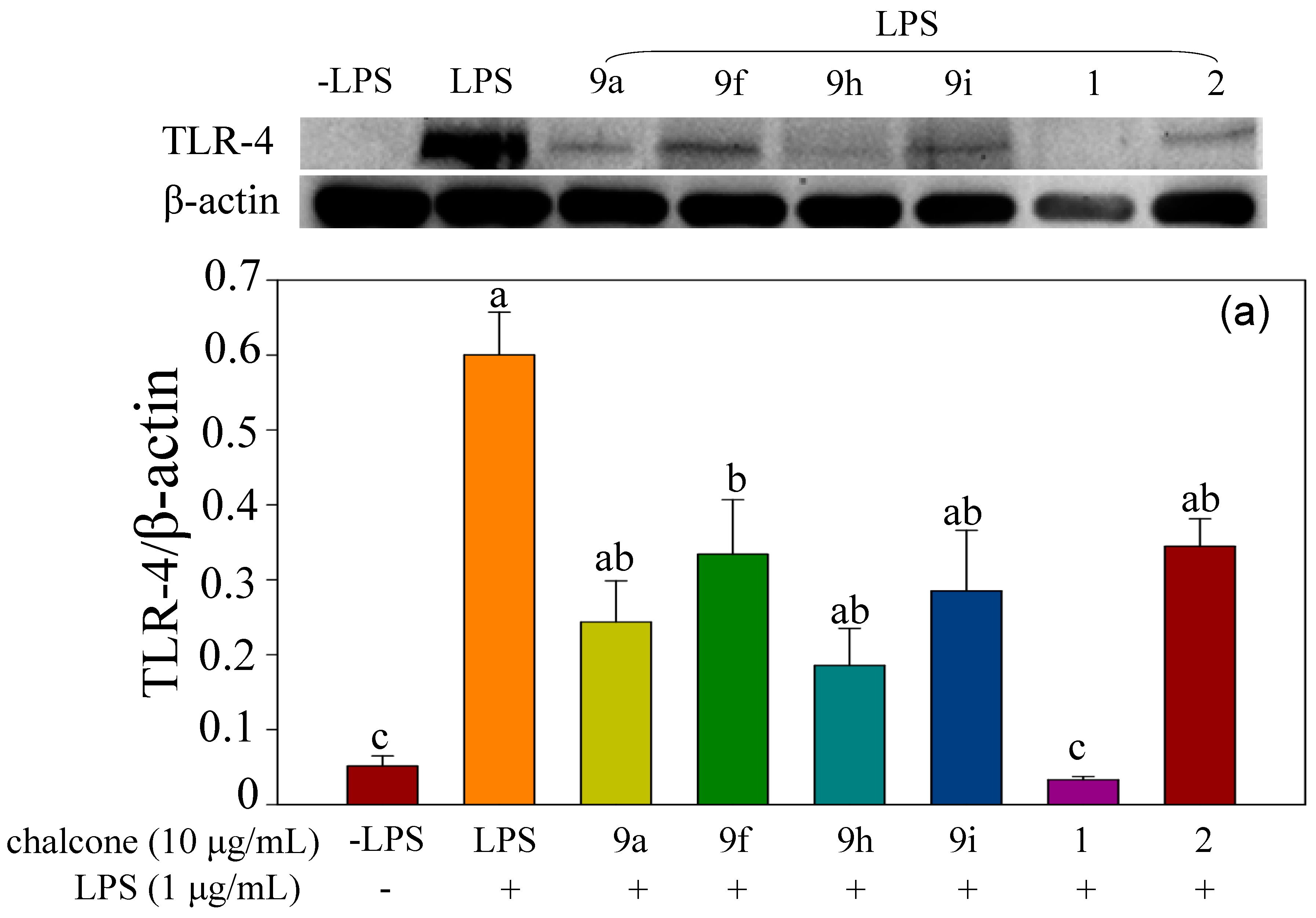
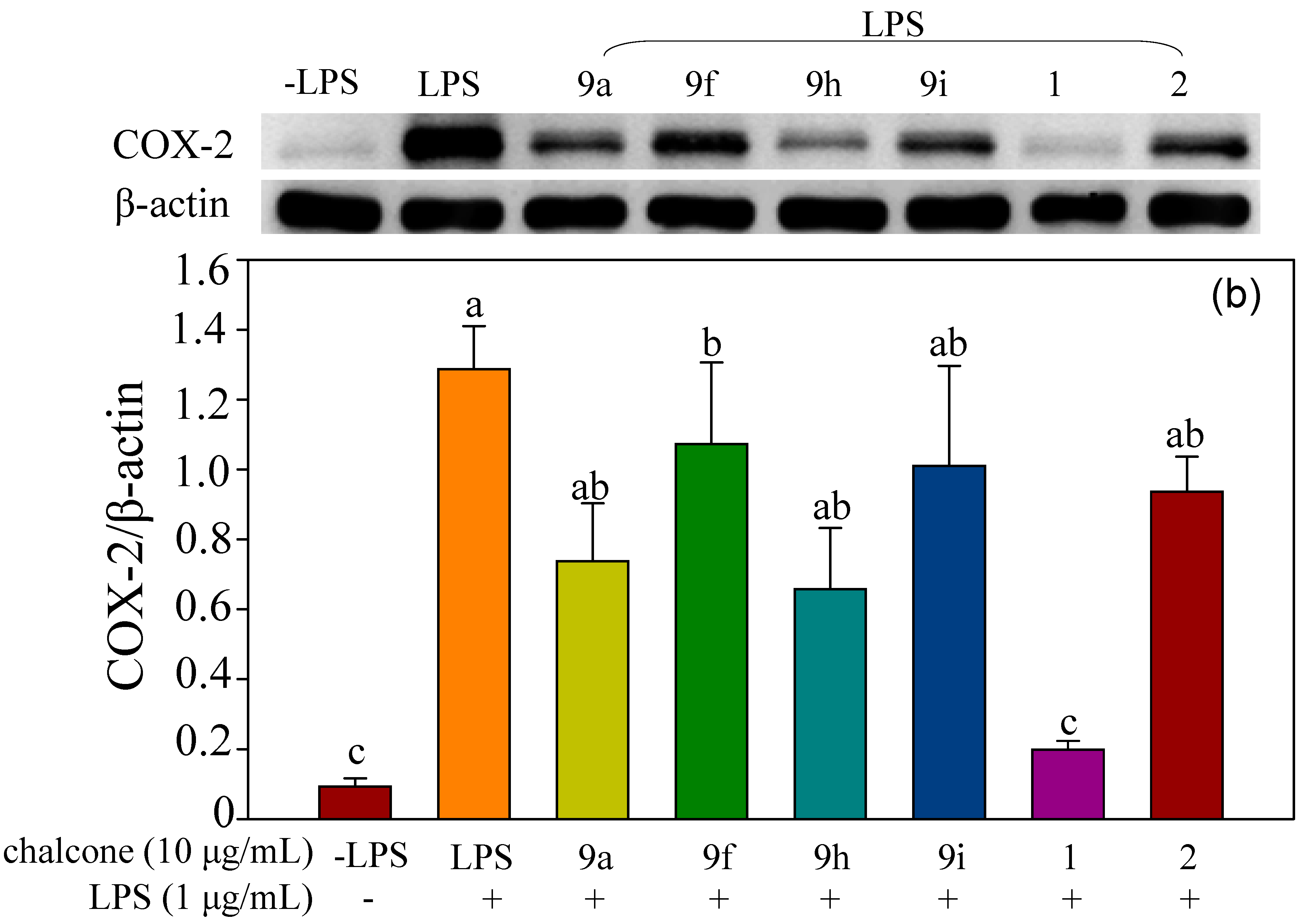
2.2.2. Effect of Halo-Substituted Chalcones (7a–i) and Azachalcones (9a–i) on mRNA and Protein Expression of Pro-Inflammatory Factors
3. Materials and Methods
3.1. General Procedure of Claisen-Schmidt Condensation in Synthesis of 7a–i
3.1.1. (E)-3-(4-Fluorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7a)
3.1.2. (E)-3-(4-Chlorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7b)
3.1.3. (E)-3-(4-Bromophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7c)
3.1.4. (E)-3-(3-Fluorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7d)
3.1.5. (E)-3-(3-Chlorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7e)
3.1.6. (E)-3-(3-Bromophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7f)
3.1.7. (E)-3-(2-Fluorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7g)
3.1.8. (E)-3-(2-Chlorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7h)
3.1.9. (E)-3-(2-Bromophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (7i)
3.2. Biological Evaluation
3.2.1. Cell Culture
3.2.2. Cell Viability Assay
3.2.3. Quantitative Real-Time PCR Analysis
3.2.4. Immunoblot Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rakoff-Nahoum, S. Why cancer and inflammation. Yale J. Biol. Med. 2006, 79, 123–130. [Google Scholar] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Med. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 2012, 18, 4266–4288. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, A.R.; O’Hagan, H.M. Interplay between inflammation and epigenetic changes in cancer. Prog. Mol. Biol. Transl. Sci. 2016, 144, 69–117. [Google Scholar] [PubMed]
- Kim, Y.-J.; Ko, H.; Park, J.-S.; Han, I.-H.; Amor, E.C.; Lee, J.W.; Yang, H.O. Dimethyl cardamonin inhibits lipopolysaccharide-induced inflammatory factors through blocking NF-κB p65 activation. Int. Immunopharmacol. 2010, 10, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.; Jantan, I.; Jasamai, M. Anti-inflammatory trends of 1,3-diphenyl-2-propen-1-one derivatives. Mini Rev. Med. Chem. 2013, 13, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Venkateswararao, E.; Sharma, V.K.; Yun, J.; Kim, Y.; Jung, S.-H. Anti-proliferative effect of chalcone derivatives through inactivation of NF-kB in human cancer cells. Bioorg. Med. Chem. 2014, 22, 3386–3392. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Deng, L.; Wang, L.; Zhang, Y.; Weng, Q.; Yin, H.; Pan, Y.; Tong, C.; Wang, J.; Liang, G. Inhibition of mitogen-activated protein kinases/nuclear factor κB-dependent inflammation by a novel chalcone protects the kidney from high fat diet-induced injuries in mice. J. Pharmacol. Exp. Ther. 2015, 355, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K. Therapeutic potential of chalcones as cardiovascular agents. Life Sci. 2016, 148, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Vogel, S.; Barbic, M.; Jürgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Prabhakar, V.; Sangith, A.; Chandrika, B.; Balasubramanian, R. Synthesis, anti-inflamatory and antioxidant activity of ring-A-monosubstituted chalcone derivatives. Med. Chem. Res. 2014, 23, 4383–4394. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D.K. Chalcone derivatives as potential antifungal agents: Synthesis, and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, S.P.; Hande, S.V.; Agrawal, N.R.; Chandak, H.S.; Bhoj, P.S.; Goswami, K.; Reddy, M.V.R. Sulfonamide chalcones: Synthesis and in vitro exploration for therapeutic potential against Brugia malayi. Eur. J. Med. Chem. 2016, 124, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Joen, K.-H.; Lee, E.; Jun, K.-Y.; Eom, J.-E.; Kwak, S.Y.; Na, Y.; Kwon, Y. Neuroprotective effect of synthetic chalcone derivative as competitive dual inhibitors against μ-calpain and cathepsin B through the downregulation of tau phosphorylation and insoluble Aβ peptide formation. Eur. J. Med. Chem. 2016, 121, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Hammuda, A.; Shalaby, R.; Rovida, S.; Edmondson, D.E.; Binda, C.; Khalil, A. Design and synthesis of novel chalcones as potent selective monoamine oxidase-B inhibitors. Eur. J. Med. Chem. 2016, 114, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kraege, S.; Stefan, K.; Juvale, K.; Ross, T.; Willmes, T.; Wiese, M. The combination of quinazoline and chalcone moieties leads to novel potent heterodimeric modulators of breast cancer resistance protein (BCRP/ABCG2). Eur. J. Med. Chem. 2016, 117, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Emami, S. Recent advances of cytotoxic chalconoids targeting tubulin polymerization: Synthesis and biological activity. Eur. J. Med. Chem. 2016, 121, 610–639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, K.; Huang, S.; Zhang, H.; Zhang, H.; Wang, Y. Organic crystals with near-infrared amplified spontaneous emissions based on 2’-hydroxychalcone derivatives: Subtle structure modification but great property change. Angew. Chem. Int. Ed. 2015, 54, 8369–8373. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Cheng, J.; Fei, Z.; Gong, K.; Liu, Z. Synthesis of chalcones via Claisen-Schmidt condensation reaction catalyzed by acyclic acidic ionic liquids. Catal. Commun. 2008, 9, 1924–1927. [Google Scholar]
- Qian, H.; Liu, D. Synthesis of chalcones via Claisen-Schmidt reaction catalyzed by sulfonic acid-functional ionic liquids. Ind. Eng. Chem. Res. 2011, 50, 1146–1149. [Google Scholar] [CrossRef]
- Otto, S.; Bertoncin, F.; Engberts, J.B.F.N. Lewis acid catalysis of a Diels-Alder reaction in water. J. Am. Chem. Soc. 1996, 118, 702–7707. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Lin, L.; Zheng, H.; Fu, K.; Liu, X.; Feng, X. N,N’-dioxide/nickel(II)-catalyzed asymmetric Diels-Alder reaction of cyclopentadiene with 2,3-dioxopyrrolidines and 2-alkenoyl pyridines. Chem. Commun. 2016, 52, 8255–8258. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.S.; Kaur, A.; Arora, R.; Dhawan, V.; Bali, M. Synthetic studies of novel azaflavanone derivatives and its biological activities. Curr. Res. Chem. 2012, 4, 88–98. [Google Scholar] [CrossRef]
- Dias, T.A.; Duarte, C.L.; Lima, C.F.; Proença, M.F.; Pereira-Wilson, C. Superior anticancer activity of halogenated chalcones and flavonols over the natural flavonol quercetin. Eur. J. Med. Chem. 2013, 65, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Kupcewicz, B.; Jarzecki, A.A.; Małecka, M.; Krajewska, U.; Rozalski, M. Cytotoxic activity of substituted chalcones in terms of molecular electronic properties. Bioorg. Med. Chem. Lett. 2014, 24, 4260–4265. [Google Scholar] [CrossRef] [PubMed]
- Batagin-Neto, A.; Lavarda, F.C. The correlation between electronic structure and antimalarial activity of alkoxylated and hydroxylated chalcones. Med. Chem. Res. 2014, 23, 580–586. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, S.; Bansal, T.; Gaba, J. Review on synthesis of bioactive pyrazoline derivatives. Chem. Sci. Trans. 2014, 3, 861–875. [Google Scholar]
- Li, C.W.; Shen, T.H.; Shih, T.-L. Reinvestigation of synthesis of halo-substituted 3-phenyl-1-(2-pyridyl)-2-propen-1-ones (azachalcones). A tandem reaction for formation of penta-substituted cyclohexanols. Tetrahedron 2017, 73, 4644–4652. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.A. The role of tumor necrosis factor in health and disease. J. Rheumatol. Suppl. 1999, 57, 16–21. [Google Scholar] [PubMed]
- Schütze, S.; Wiegmann, K.; Machleidt, T.; Krönke, M. TNF-induced activation of NF-κB. Immunobiology 1995, 193, 193–203. [Google Scholar] [CrossRef]
- Agarwal, S.; Reddy, G.V.; Reddanna, P. Eicosanoids in inflammation and cancer: The role of COX-2. Expert Rev. Clin. Immunol. 2009, 5, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Kishimoto, T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr. Opin. Pharmacol. 2004, 4, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants—past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, A.; Ottani, A.; Sandrini, M. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: Critical remarks. Curr. Med. Chem. 2002, 9, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Chaudhary, D.; Pathak, U.; Saxena, N.; Dhaked, R.K. In silico discovery and validation of amide based small molecule targeting the enzymatic site of shiga toxin. J. Med. Chem. 2016, 59, 10763–10773. [Google Scholar] [CrossRef] [PubMed]
- Rajput, J.K.; Kaur, G. Silicotungstic acid catalysed Claisen Schmidt condensation reaction: An efficient protocol for synthesis of 1,3-diaryl-2-propenones. Tetrahedron Lett. 2012, 53, 646–649. [Google Scholar] [CrossRef]
- Chen, F.C.; Yang, C.H. Haloflavones. Taiwan Pharm. J. 1951, 3, 39–41. [Google Scholar]
- Lu, M.Y.; Chen, C.C.; Lee, L.Y.; Lin, T.W.; Kuo, C.F. N6-(2-hydroxyethyl)-adenosine in medicinal mushroom Cordyceps cicadae attenuates lipopolysaccharide-stimulated pro-inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathways. J. Nat. Prod. 2015, 78, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

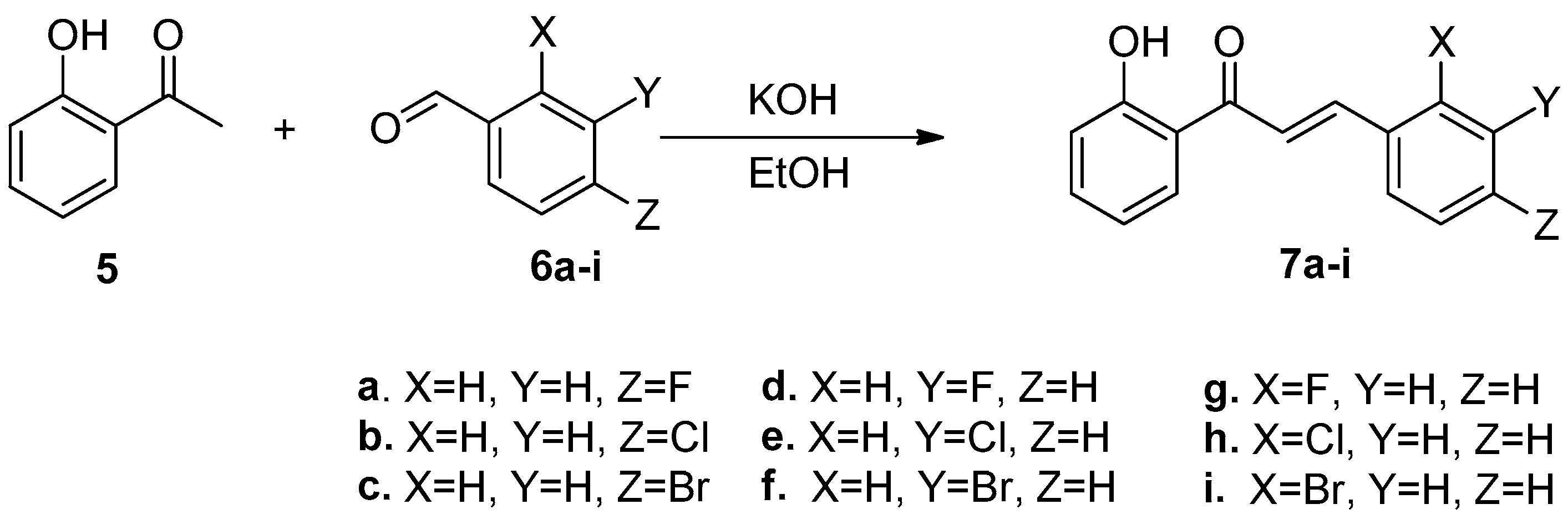
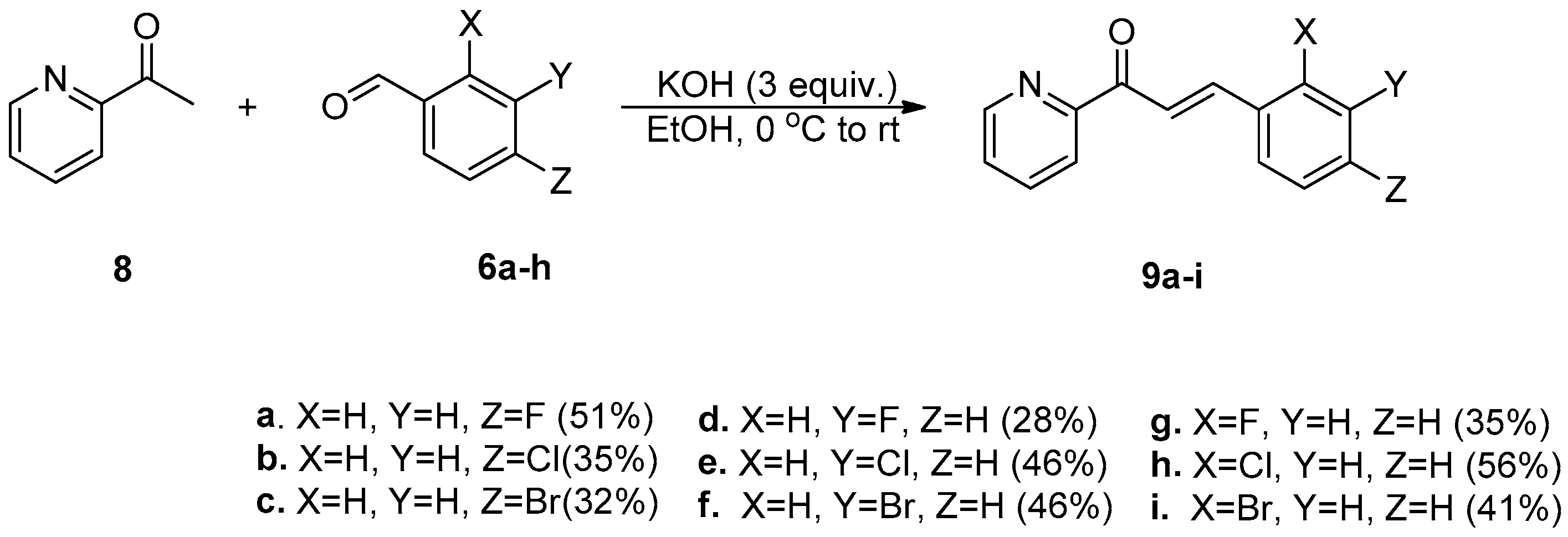
| Entry | Compound | Time (h) | Yield% |
|---|---|---|---|
| 1 | 7a | 14 | 92 |
| 2 | 7b | 9 | 75 |
| 3 | 7c | 15 | 82 |
| 4 | 7d | 8 | 76 |
| 5 | 7e | 13 | 67 |
| 6 | 7f | 14 | 73 |
| 7 | 7g | 13 | 85 |
| 8 | 7h | 14 | 78 |
| 9 | 7i | 15 | 73 |
| Compound | Concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| 0 | 10 | 25 | 50 | 100 | |
| 7a | 100.0 | 114.27 ± 4.83 1 | 99.76 ± 18.08 | 95.02 ± 21.09 | 80.32 ± 15.54 |
| 7b | 100.0 | 83.55 ± 12.07 | 105.08 ± 14.37 | 90.34 ± 19.48 | 55.51 ± 4.21 |
| 7c | 100.0 | 110.86 ± 29.05 | 115.31 ± 15.25 | 79.03 ± 6.30 | 65.77 ± 12.03 |
| 7d | 100.0 | 97.37 ± 20.37 | 86.35 ± 18.47 | 71.78 ± 10.27 | 34.72 ± 4.15 |
| 7e | 100.0 | 83.42 ± 13.10 | 69.68 ± 5.17 | 45.95 ± 6.08 | 17.24 ± 1.29 |
| 7f | 100.0 | 71.29 ± 8.12 | 56.92 ± 4.88 | 30.60 ± 4.96 | 19.32 ± 2.89 |
| 7g | 100.0 | 80.47 ± 8.38 | 75.60 ± 12.85 | 68.21 ± 7.45 | 42.19 ± 4.87 |
| 7h | 100.0 | 101.07 ± 8.63 | 87.16 ± 9.03 | 60.71 ± 7.09 | 16.35 ± 0.56 |
| 7i | 100.0 | 87.00 ± 18.69 | 64.16 ± 6.38 | 32.19 ± 3.32 | 24.23 ± 2.00 |
| 9a | 100.0 | 99.00 ± 3.19 | 93.66 ± 3.87 | 70.68 ± 2.17 | 4.22 ± 0.47 |
| 9b | 100.0 | 123.61 ± 0.10 | 112.57 ± 8.72 | 82.18 ± 3.87 | 3.92 ± 0.19 |
| 9c | 100.0 | 93.28 ± 9.09 | 69.54 ± 23.80 | 14.51 ± 2.05 | 5.52 ± 0.37 |
| 9e | 100.0 | 90.39 ± 10.15 | 85.06 ± 8.59 | 63.56 ± 3.03 | 8.60 ± 1.06 |
| 9f | 100.0 | 102.57 ± 16.87 | 71.97 ± 8.07 | 50.11 ± 9.90 | 15.27 ± 1.64 |
| 9g | 100.0 | 86.56 ± 12.35 | 89.25 ± 5.68 | 34.25 ± 3.08 | 3.3 ± 0.22 |
| 9h | 100.0 | 84.49 ± 8.66 | 86.32 ± 8.10 | 60.61 ± 25.05 | 43.90 ± 18.91 |
| 9i | 100.0 | 79.14 ± 11.53 | 77.95 ± 10.43 | 67.49 ± 8.26 | 33.68 ± 5.03 |
| 0 | 1 | 5 | 10 | 25 | |
| 1 | 100.0 | 105.09 ± 12.45 | 103.04 ± 5.41 | 75.05 ± 7.95 | 23.64 ± 4.82 |
| 2 | 100.0 | 99.80 ± 14.60 | 102.52 ± 10.87 | 87.14 ± 14.13 | 51.99 ± 6.98 |
| 3 | 100.0 | 86.98 ± 6.15 | 81.19 ± 8.43 | 64.09 ± 4.80 | 34.01 ± 4.21 |
| 4 | 100.0 | 90.63 ± 16.23 | 103.44 ± 8.52 | 85.54 ± 12.25 | 45.88 ± 6.48 |
| Gene | Forward Primer 5′3′ | Reverse Primer 5′3′ |
|---|---|---|
| SRP72 | CACACCCTAGCCCAACTTATT | TCAAGCGCCTCAACATCTAC |
| TLR4 | TTCAGAACTTCAGTGGCTGGATTTA | GTCTCCACAGCCACCAGATTCTC |
| IκB-α | CCTTCCTCAACTTCCAGAACAA | GATCACAGCCAGCTTTCAGA |
| p65 | TGTGGAGATCATCGAACAGCCGAA | TGTTCCTGGTCCTGTGTAGCCATT |
| IL-1β | GTTACATCAGCACCTCACAA | TTAGAAACAGCTCAGCCCATAC |
| IL-6 | CTTCCATCCAGTTGCCTTCT | CTCCGACTTGTGAAGTGGTATAG |
| TNF-α | TTGTCTACTCCCAGGTTCTCT | GAGGTTGACTTTCTCCTGGTATG |
| COX-2 | CGGACTGGATTCTATGGTGAAA | CTTGAAGTGGGTCAGGATGTAG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, T.-L.; Liu, M.-H.; Li, C.-W.; Kuo, C.-F. Halo-Substituted Chalcones and Azachalcones Inhibited Lipopolysaccharited-Stimulated Pro-Inflammatory Responses through the TLR4-Mediated Pathway. Molecules 2018, 23, 597. https://doi.org/10.3390/molecules23030597
Shih T-L, Liu M-H, Li C-W, Kuo C-F. Halo-Substituted Chalcones and Azachalcones Inhibited Lipopolysaccharited-Stimulated Pro-Inflammatory Responses through the TLR4-Mediated Pathway. Molecules. 2018; 23(3):597. https://doi.org/10.3390/molecules23030597
Chicago/Turabian StyleShih, Tzenge-Lien, Ming-Hwa Liu, Chia-Wai Li, and Chia-Feng Kuo. 2018. "Halo-Substituted Chalcones and Azachalcones Inhibited Lipopolysaccharited-Stimulated Pro-Inflammatory Responses through the TLR4-Mediated Pathway" Molecules 23, no. 3: 597. https://doi.org/10.3390/molecules23030597