Novel Trifluoromethylcoumarinyl Urea Derivatives: Synthesis, Characterization, Fluorescence, and Bioactivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. UV Absorption and Fluorescence Characteristics
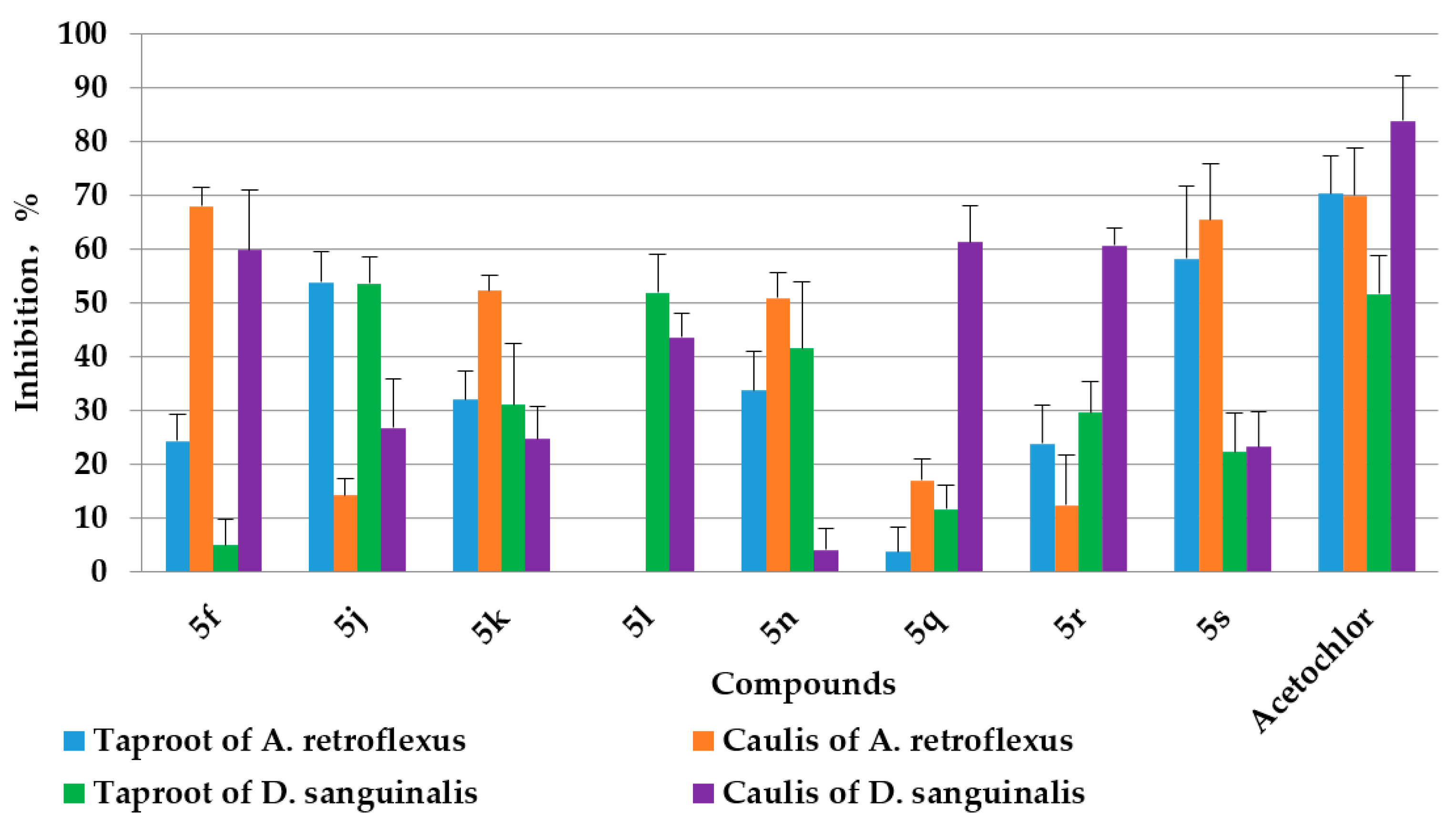
2.3. Herbicidal Activities
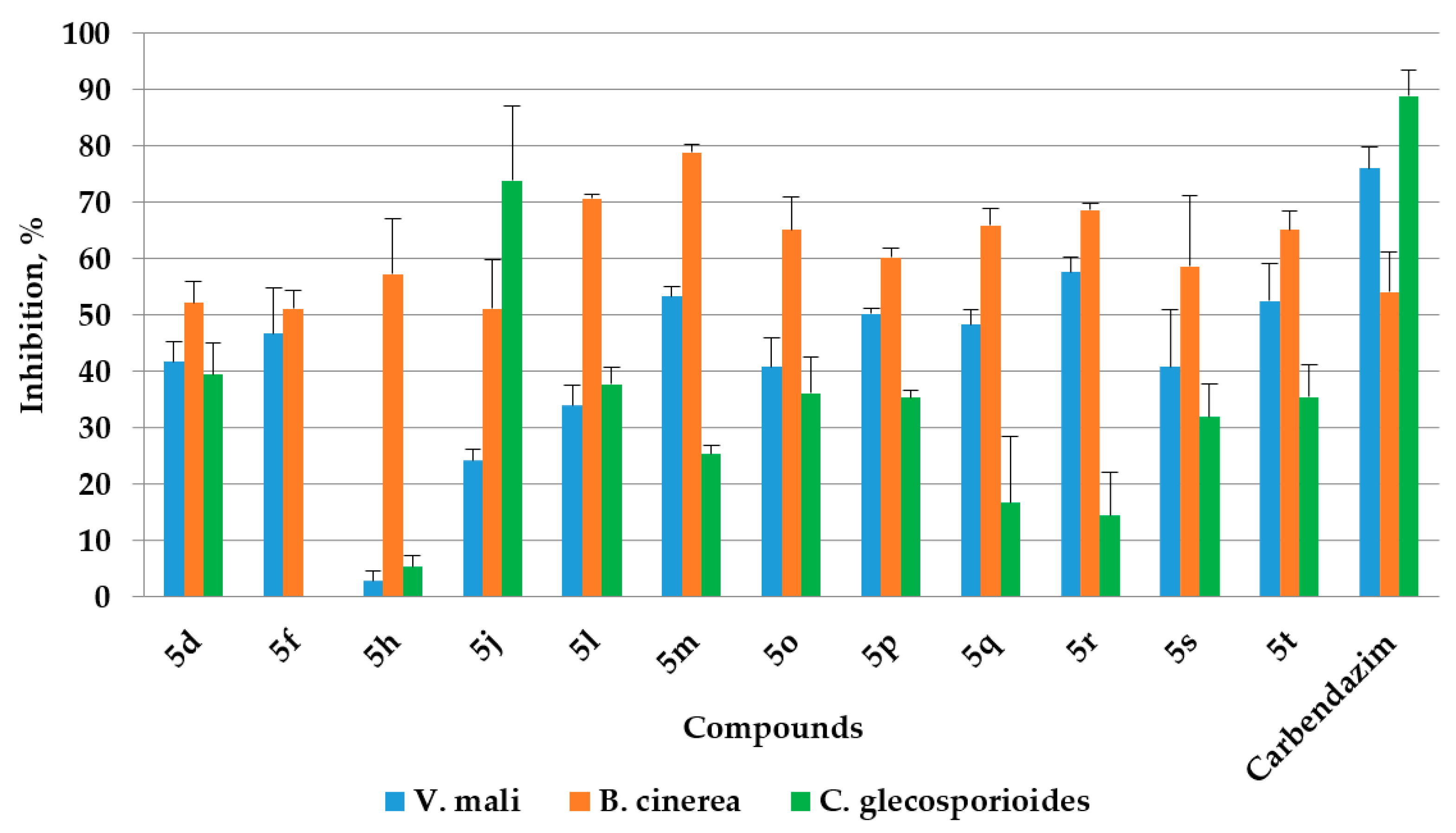
2.4. Antifungal Activities
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedures
3.1.2. Synthesis of 4-Trifluoromethyl-7-hydroxycoumarin (1)
3.1.3. Synthesis of 4-Trifluoromethyl-7-methoxycoumarin (2)
3.1.4. Synthesis of 4-Trifluoromethyl-6-nitro-7-methoxycoumarin (3)
3.1.5. Synthesis of 4-Trifluoromethyl-6-amino-7-methoxycoumarin (4)
3.1.6. General Procedure for Synthesis of 4-Trifluoromethyl-6-amino-7-methoxycoumarinyl Urea Derivatives (5a–5u)
3.2. Determination of UV Absorption and Fluorescence Spectrum
3.3. Procedures for Activities Evaluation
3.3.1. Herbicidal Activity
3.3.2. Antifungal Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Umadevi, P.; Deepti, K.; Srinath, I.; Vijayalakshmi, G.; Tarakaramji, M. Synthesis and in-vitro antibacterial activity of some new urea, thiourea and thiosemicarbazide derivatives. Int. J. Pharm. Pharm. Sci. 2012, 4, 379–383. [Google Scholar]
- Rajtar, B.; Szacon, E.; Swiatek, L.; Rzadkowska, M.; Matosiuk, D.; Mal-gorzata, P.D. Antiviral activity of 1-(1-arylimidazolidine-2-ylidene)-3-(4-chlorobenzyl)urea derivatives. J. Pre-Clin. Clin. Res. 2013, 7, 104–106. [Google Scholar]
- Venkatachalam, T.K.; Mão, C.; Uckun, F.M. Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 2004, 12, 4275–4284. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, R.K.; Petruschke, R.A. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J. Antimicrob. Chemother. 2004, 53, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Sarantakis, D.; Terpinski, J.; Kumar, T.R.; Tsai, H.C.; Kuo, M.; Ager, A.L.; Jacobs, J.W.R.; Schiehser, G.A.; Ekins, S.; et al. Novel diaryl ureas with efficacy in a mousemodel of malaria. Bioorg. Med. Chem. Lett. 2013, 23, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; EI-Gamal, M.I.; Oh, C.H.; Lee, S.H.; Kim, G.; Jun, H.H.; Hong, S.C.; Kyung, H.Y. Synthesis and antiproliferative activity of new aminoisoquinolinylurea derivatives against melanoma cell line. Bull. Korean Chem. Soc. 2012, 33, 3635–3639. [Google Scholar] [CrossRef]
- Lu, C.S.; Tang, K.; Li, Y.; Jin, B.; Yin, D.L.; Chen, X.G.; Ma, C.; Huang, H.H. Synthesis and in vitro antitumor activities of novel benzyl urea analogues of sorafenib. Yao Xue Xue Bao 2013, 48, 709–717. [Google Scholar] [PubMed]
- Mathieu, G.B.; Sébastien, F.; Jacques, L.; Lefebvre, C.A.; Côté, M.F.; René, C.G. Styryl-N-phenyl-N’-(2-chloroethyl)ureas and styrylphenylimidazolidin-2-ones as new potent microtubule-disrupting agents using combretastatin A-4 as model. Eur. J. Med. Chem. 2015, 100, 34–43. [Google Scholar]
- Nimbarte, V.D.; Murtuza, H.; Phaniraj, S.; Shrivastava, S.; Naidu, V.G.M.; Kumar, N.S.; Atcha, K.R. Design, synthesis and biological evaluation of 4-(1-(4(sulphanilamide)phenyl)-3-(methyl)-1H-pyrazol-5-yl)dine urea and N-acyl derivatives as a soluble epoxide hydrolase inhibitors. Med. Chem. Res. 2014, 23, 2178–2197. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Bongu, S.; Gomathi, P. Anti-ulcerogenic activity of unsymmetrically substituted urea derivative (1-benzyl-3-(4-methylphenyl) urea. Afr. J. Pharm. Pharmacol. 2013, 7, 2522–2527. [Google Scholar] [CrossRef]
- Ramesh, D.; Gangadhar, S.; Katti, S.A. Synthesis, characterization and anticonvulsant activity of 1-(aryl)-3-(diphenylmethyl) urea derivatives. Int. J. Res. Pharm. Chem. 2013, 3, 748–752. [Google Scholar]
- Lorena, S.; Lima, L.A.; Valdir, C.F.; Rogério, C.; Fátima, C.B.; Nunes, R.J. Synthesis of new 1-phenyl-3-{4-[(2E)-3-phenylprop-2-enoyl]phenyl}-thiourea and urea derivatives with anti-nociceptive activity. Bioorg. Med. Chem. 2008, 16, 8526–8534. [Google Scholar]
- Mishra, C.B.; Kumari, S.; Tiwari, M. Design and synthesis of some new 1-phenyl-3/4-[4-(aryl/heteroaryl/alkyl-piperazine1-yl)-phenyl-ureas as potent anticonvulsant and antidepressant agents. Arch. Pharm. Res. 2016, 39, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, X.Y.; Tang, W.; Groeneveld, T.W.L.; He, P.L.; Zhu, F.H.; Li, J.; Lu, W.; Blom, A.M.; Zuo, J.P.; et al. Discovery and Structural Modification of 1-Phenyl-3-(1-phenylethyl)urea Derivatives as Inhibitors of Complement. ACS Med. Chem. Lett. 2012, 3, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Furman, C.; Lebeau, J.; Fruchart, J.C.; Bernier, J.L.; Duriez, P.; Cotelle, N.; Teissier, E. Di-tert-butylhydroxylated flavonoids protect endothelial cells against oxidized LDL-induced cytotoxicity. J. Biochem. Mol. Toxicol. 2001, 15, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ryu, J.H.; Cheon, Y.J.; Lim, H.J.; Jeon, R. Design and synthesis of urea and thiourea derivatives and their inhibitory activities on lipopolysaccharide-induced NO production. Bioorg. Med. Chem. Lett. 2007, 17, 3317–3321. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.X.; Song, H.J.; Li, Y.Q.; Wang, Q.M. Design, synthesis, insecticidal activity, and structure-activity relationship (SAR): Studies of novel triazone derivatives containing a urea bridge group based on transient receptor potential (TRP) channels. Mol. Divers. 2016, 20, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.R.; Oulkar, D.P.; Shabeer, T.P.A.; Banerjee, K. Quantitative screening of agrochemical residues in fruits and vegetables by buffered ethyl acetate extraction and LC-MS/MS analysis. J. Agric. Food Chem. 2015, 63, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- George, L. Herbicidal Sulfonamide Combinations. Patent DE2853561A1, 8 May 1980. [Google Scholar]
- Zhang, Z.W.; Yang, H.H.; Gao, Z.H.; Yuan, Y.H.; Dong, J.; Wang, Y.; Yue, T.L. Identification, Synthesis, and Safety Assessment of Thidiazuron [1-Phenyl-3-(1,2,3-thidiazol-5-yl) urea] and Its Metabolites in Kiwifruits. J. Agric. Food Chem. 2017, 65, 11273–11279. [Google Scholar] [CrossRef] [PubMed]
- Schiedel, M.S.; Briehn, C.A.; Baüerle, P. Single-compound libraries of organic materials: Parallel synthesis and screening of fluorescent dye. Angew. Chem. Int. Ed. 2001, 40, 4677–4680. [Google Scholar] [CrossRef]
- Swanson, S.A.; Wallraff, G.M.; Chen, J.P.; Zhang, W.; Bozano, L.D.; Carter, K.R.; Salem, J.R.; Villa, R.; Scott, J.C. Stable and efficient fluorescent red and green dyes for external and internal conversion of blue OLED emission. Chem. Mater. 2003, 15, 2305–2312. [Google Scholar] [CrossRef]
- Lee, M.T.; Yen, C.K.; Yang, W.P.; Chen, H.H.; Liao, C.H.; Tsai, C.H.; Chen, C.H. Efficient green coumarin dopants for organic light-emitting devices. Org. Lett. 2004, 6, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhang, P.; Zhao, Y.; Zhang, H.; Meng, J.; Fan, D. Synthesis, characterization and high-efficiency blue electroluminescence based on coumarin derivatives of 7-diethylamino-coumarin-3-carboxamide. Org. Electron. 2009, 10, 653–660. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Zhou, Y.; Gao, W.; Song, H.; Wang, X.; Wang, Y. A highly selective and sensitive fluorescence probe for rapid detection of hypochlorite in tap water and cancer cells. RSC Adv. 2016, 6, 105795–105800. [Google Scholar] [CrossRef]
- Wu, C.J.; Wang, J.B.; Shen, J.J.; Bi, C.; Zhou, H.W. Coumarin-based Hg2+ fluorescent probe: Synthesis and turn-on fluorescence detection in neat aqueous solution. Sens. Actuators B Chem. 2017, 243, 678–683. [Google Scholar] [CrossRef]
- Paul, M.K.; Singh, Y.D.; Dey, A.; Saha, S.K.; Anwar, S.; Chattopadhyay, A.P. Coumarin based emissive rod shaped new Schiff base mesogens and their Zinc(II) complexes: Synthesis, photophysical, meso-morphism, gelation and DFT studies. Liq. Cryst. 2016, 43, 343–360. [Google Scholar] [CrossRef]
- Zhou, W.J.; Sun, X.Q.; Xi, H.T. Synthesis and fluorescence properties of hyaluronic acid derivatives containing fluorinated coumarin. Chin. J. Appl. Chem. 2014, 31, 916–921. [Google Scholar]
- Ostrowska, K.; Młodzikowska, K.; Głuch-Lutwin, M.; Gryboś, A.; Siwek, A. Synthesis of a new series of aryl/heteroarylpiperazinyl derivatives of 8-acetyl-7-hydroxy-4-methylcoumarin with low nanomolar 5-HT 1A affinities. Eur. J. Med. Chem. 2017, 137, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Xue, Y.B.; Liu, Z.; Peng, S.S.; He, Y.; Zhang, Y.; Fang, R.; Wang, J.P.; Luo, Z.W.; Yao, G.M.; et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 2015, 5, 13544. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.; Reem, I.A. Anti-inflammatory screening and molecular modeling of some novel coumarin derivatives. Molecules 2015, 20, 5374–5391. [Google Scholar]
- Cong, N.T.; Nhan, H.T.; Van, H.L.; Thang, T.D.; Kuo, P.C. Synthesis and antibacterial activity of analogs of 5-arylidene-3-(4-methylcoumarin-7-yloxyacetylamino)-2-thioxo-1,3-thiazoli-din-4-one. Molecules 2014, 19, 13577–13586. [Google Scholar] [CrossRef] [PubMed]
- Šarkanj, B.; Molnar, M.; Cacic, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 139, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Bonacorso, H.G.; Rodrigues, M.B.; Rosa, W.C.; Silva, L.B.; Frizzo, C.P.; Zanatta, N.; Martins, M.A.P. Cyanoacetylazoles and salicylic aldehydes promoting the synthesis of new trifluoromethyl-substituted azolecarbonyl-2H-chromen-2-ones through the Knoevenagel condensation reaction. J. Fluorine Chem. 2015, 178, 296–305. [Google Scholar] [CrossRef]
- Liu, G.L.; Hu, Y.; Chen, X.H.; Wang, G.X.; Ling, F. Synthesis and anthelmintic activity of coumarin- imidazole hybrid derivatives against Dactylogyrus intermedius in goldfish. Bioorg. Med. Chem. Lett. 2016, 26, 5039–5043. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, X.Z.; Yan, Z.Q.; Guo, H.R.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure-activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, X.; Jin, H.; Yang, X.; Qin, B. Antifungal activity of umbelliferone derivatives: Synthesis and structure-activity relationships. Microb. Pathog. 2017, 104, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Pawar, P.; Joseph, M.; Phalgune, M.; Kashalkar, U.; Deshpande, R.; Nirmala, R. Efficacy of 4-methy-7-hydroxy coumarin derivatives against vectors Aedes aegypti and Culex quinquefasciatus. Indian J. Exp. Biol. 2008, 46, 788–792. [Google Scholar]
- Chetan, T.P.; Sangamesh, A.P.; Ajaykumar, D.K.; Vinod, H.N.; Manjunathad, M.; Shivshankar, M.K.; Prema, S.B. Synthesis, spectral, thermal, fluorescence, antimicrobial, anthelmintic and DNA cleavage studies of mononuclear metal chelates of bi-dentate 2H-chromene-2-one Schiff base. J. Photochem. Photobiol. B 2015, 148, 322–332. [Google Scholar]
- Wei, Y.; Miao, K.L.; Hao, S.H. Novel 4-methylumbelliferone amide derivatives: Synthesis, characterization and pesticidal activities. Molecules 2018, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Chaabouni, S.; Simonet, F.; François, A.; Abid, S.; Galaup, C.; Chassaing, S. 3-Trifluoromethylated coumarins and carbostyrils via radical trifluoromethylation of ortho-functionalized cinnamic esters. Eur. J. Org. Chem. 2017, 2017, 271–277. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, B.V.; Lantaño, B.; Postigo, A. Recent advances in trifluoromethylation reactions with electrophilic trifluoromethylating reagents. Chem. Eur. J. 2014, 20, 16806–16829. [Google Scholar]
- Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Acena, J.L.; Izawa, K.; Liu, H.; Soloshonok, V.A. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluorine Chem. 2014, 167, 37–54. [Google Scholar] [CrossRef]
- Kees, K.L.; Fitzgerald, J.J.; Steiner, K.E.; Mattes, J.F.; Mihan, B.; Tosi, T.; Mondoro, D.; McCalebr, M.L. New potent antihyperglycemic agents in db/db mice: Synthesis and structure−activity relationship studies of (4-substituted benzyl) (trifluoromethyl) pyrazoles and -pyrazolones. J. Med. Chem. 1996, 39, 3920–3928. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Luzina, E.L.; Popov, A.V. Synthesis and anticancer activity of N-bis(trifluoromethyl)alkyl-N’-thiazolyl and N-bis(trifluoromethyl)alkyl-N’-benzothiazolyl ureas. Eur. J. Med. Chem. 2009, 44, 4944–4953. [Google Scholar] [CrossRef] [PubMed]
- Luzina, E.L.; Popov, A.V. Anticancer activity of N-bis(trifluoromethyl)alkyl-N’-(polychlorophenyl) and N’-(1,2,4-triazolyl) ureas. Eur. J. Med. Chem. 2010, 45, 5507–5512. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Lin, Y.; Rodrigues, A.D.; Rushmore, T.H.; Baillie, T.A.; Shou, M. Testosterone, 7-benzyloxyquinoline, and 7-benzyloxy-4-trifluoromethyl-coumarin bind to different domains within the active site of cytochrome P450 3A4. Drug. Metab. Dispos. 2001, 29, 1473–1479. [Google Scholar] [PubMed]
- Liu, J.; Shah, M.B.; Zhang, Q.; Stout, C.D.; Halpert, J.R.; Wilderman, P.R. Coumarin derivatives as substrate probes of mammalian cytochromes P450 2B4 and 2B6: Assessing the importance of 7-alkoxy chain length, halogen substitution, and non-active site mutations. Biochemistry 2016, 55, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.W.; Eley, B.M. Preliminary studies on cysteine and serine proteinase activities in inflamed human gingiva using different 7-amino-4-trifluoromethyl coumarin substrates and protease inhibitors. Arch. Oral Biol. 1987, 32, 599–605. [Google Scholar] [CrossRef]
- Qiao, L.L.; Wei, Y.; Hao, S.H. Synthesis and Biological Activity of Novel Fluorinated Amide Hydroxy Methyl Coumarin Derivatives. Chin. J. Org. Chem. 2018, 38, 509–518. [Google Scholar] [CrossRef]
- Wang, H.S. Magnesium bis (trifluoromethane) sulfonimide: An efficient catalyst for the synthesis of coumarins under solvent-free conditions. Monatshte Fur Chem. 2013, 144, 411–414. [Google Scholar] [CrossRef]
- DeGrote, J.; Tyndall, S.; Wong, K.F.; VanAlstine-Parris, M. Synthesis of 7-alkoxy-4-trifluoromethylcoumarins via the von Pechmann reaction catalyzed by molecular iodine. Tetrahedron Lett. 2014, 55, 6715–6717. [Google Scholar] [CrossRef]
- Ci, Y.X.; Jia, X.A. Simplified method of measuring fluorescence quantum efficiency. Anal. Chem. 1986, 8, 616–618. [Google Scholar]
- Wei, Y.; Li, S.Q.; Hao, S.H. New angular oxazole-fused coumarin derivatives: Synthesis and biological activities. Nat. Prod. Res. 2017, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

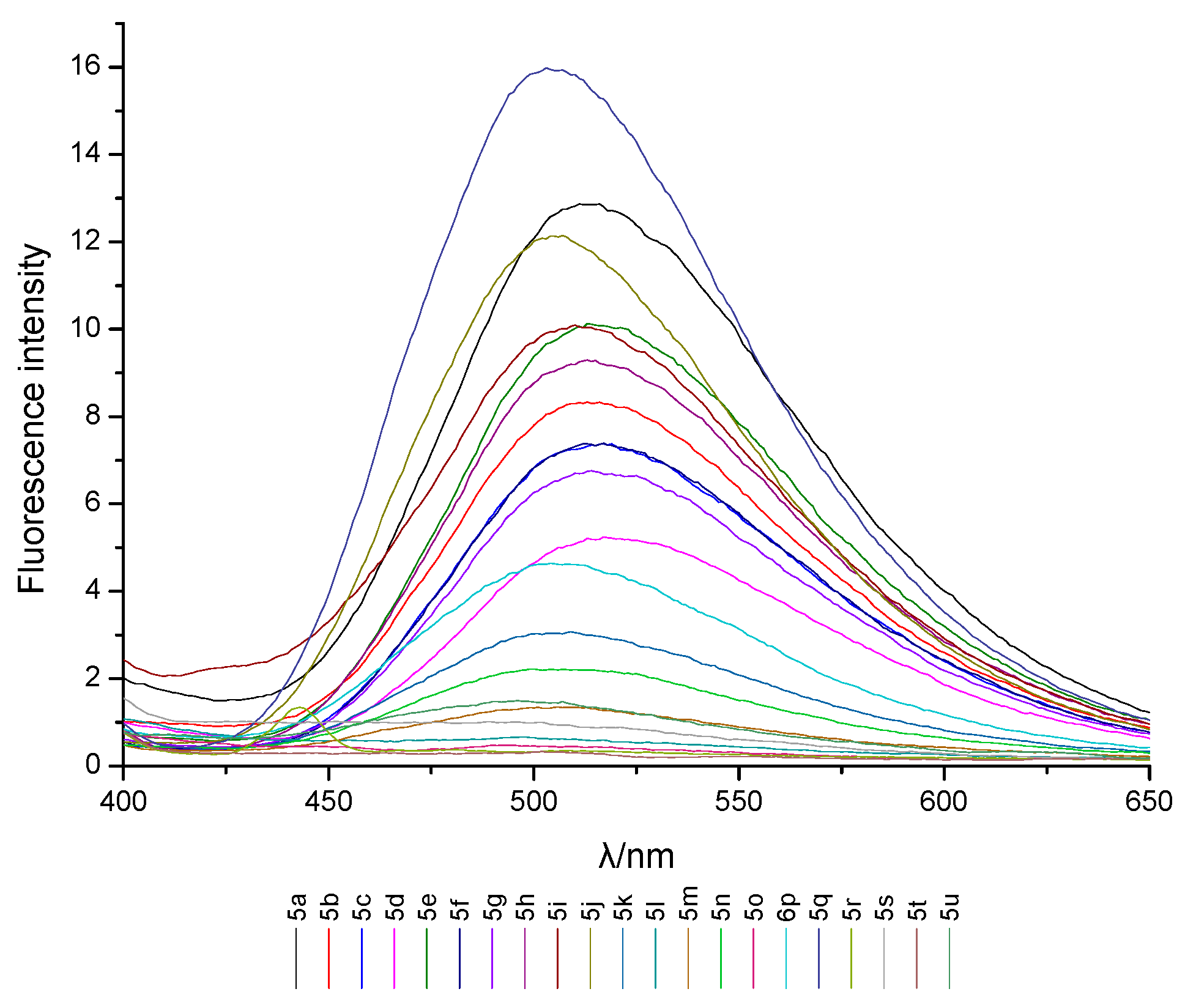
| Compd. | λmaxab (nm) | A | εmax × 103 (L·mol−1·cm−1) | λmaxem (nm) | Δλ (nm) | Φf |
|---|---|---|---|---|---|---|
| 5a | 369 | 0.216 | 7.13 | 512 | 143 | 0.114 |
| 5b | 369 | 0.186 | 6.40 | 511 | 142 | 0.110 |
| 5c | 374 | 0.180 | 6.19 | 516 | 143 | 0.065 |
| 5d | 373 | 0.188 | 6.73 | 517 | 145 | 0.044 |
| 5e | 360 | 0.189 | 7.30 | 513 | 154 | 0.087 |
| 5f | 369 | 0.160 | 5.92 | 512 | 144 | 0.064 |
| 5g | 370 | 0.154 | 5.92 | 513 | 143 | 0.053 |
| 5h | 370 | 0.150 | 6.90 | 512 | 142 | 0.070 |
| 5i | 366 | 0.129 | 5.24 | 510 | 144 | 0.082 |
| 5j | 367 | 0.182 | 7.50 | 507 | 141 | 0.098 |
| 5k | 367 | 0.171 | 7.05 | 509 | 143 | 0.024 |
| 5l | 369 | 0.174 | 7.17 | 381 | 12 | 0.028 |
| 5m | 363 | 0.162 | 6.71 | 506 | 143 | 0.013 |
| 5n | 367 | 0.183 | 7.25 | 500 | 134 | 0.020 |
| 5o | 367 | 0.164 | 6.43 | - | - | - |
| 5p | 363 | 0.166 | 7.40 | 504 | 141 | 0.041 |
| 5q | 367 | 0.134 | 5.98 | 503 | 137 | 0.115 |
| 5r | 372 | 0.143 | 5.84 | 443 | 72 | 0.005 |
| 5s | 363 | 0.134 | 5.47 | 396 | 33 | 0.013 |
| 5t | 365 | 0.136 | 5.55 | - | - | - |
| 5u | 367 | 0.166 | 7.67 | 387 | 21 | 0.013 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, L.; Hao, S. Novel Trifluoromethylcoumarinyl Urea Derivatives: Synthesis, Characterization, Fluorescence, and Bioactivity. Molecules 2018, 23, 600. https://doi.org/10.3390/molecules23030600
Qiao L, Hao S. Novel Trifluoromethylcoumarinyl Urea Derivatives: Synthesis, Characterization, Fluorescence, and Bioactivity. Molecules. 2018; 23(3):600. https://doi.org/10.3390/molecules23030600
Chicago/Turabian StyleQiao, Lili, and Shuanghong Hao. 2018. "Novel Trifluoromethylcoumarinyl Urea Derivatives: Synthesis, Characterization, Fluorescence, and Bioactivity" Molecules 23, no. 3: 600. https://doi.org/10.3390/molecules23030600





