Multi-Target Anti-Alzheimer Activities of Four Prenylated Compounds from Psoralea Fructus
Abstract
:1. Introduction
2. Results and Discussion
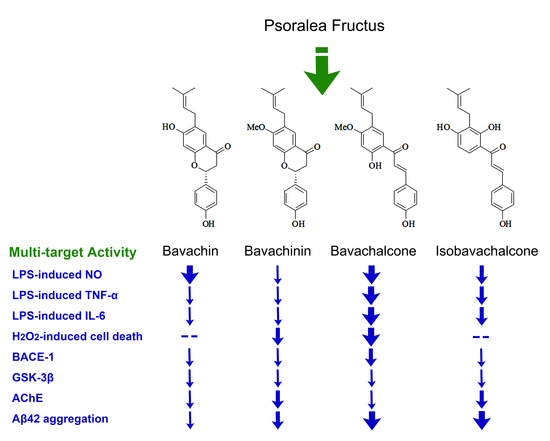
2.1. Chemical Structures of the Purified Compounds
2.2. Anti-Neuroinflammatory Effect in BV-2 Microglia
2.3. Anti-Oxidative Effects in PC-12 Cells
2.4. Inhibitory Effects on BACE-1, GSK-3β, and AChE
2.5. Inhibitory Effects on Aggregation of Aβ42
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Chemicals and Reagents
3.4. Isolation of Compounds from the Fruits of P. corylifolia
3.5. Compound Characterization
3.6. Anti-Neuroinflammatory Effect in LPS-induced BV-2 Microglia
3.6.1. Inhibition Assay on NO Release
3.6.2. Inhibition Assay on Cytokine Release
3.7. Anti-Oxidative Effect in H2O2-Induced PC-12 Cells
3.8. Enzyme Inhibition Assays of BACE-1, GSK-3β and AChE
3.9. Anti-Aggregation Assay of Aβ42
3.10. Docking Studies of Aβ42 Monomer
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2015; Volume I, pp. 187–188. ISBN 978-7-5067-7337-9. [Google Scholar]
- Zhao, L.; Wu, M.; Xiang, B. Analysis of Psoralea corylifolia L. fruits in different regions. Chem. Pharm. Bull. 2005, 53, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Zhao, W.W.; Wang, Y.; Lu, J.J.; Chen, X.P. The chemical constituents and bioactivities of Psoralea corylifolia Linn.: A review. Am. J. Chin. Med. 2016, 44, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.M.; Taniguchi, S.; Kuroda, T.; Hatano, T. Constituents of Psoralea corylifolia fruits and their effects on methicillin-resistant Staphylococcus aureus. Molecules 2015, 20, 12500–12511. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Wang, S.; Wang, M.; Fu, W.; Zhang, C.; Xu, D. Isobavachalcone attenuates MPTP-induced Parkinson’s disease in mice by inhibition of microglial activation through NF-κB Pathway. PLoS ONE 2017, 12, e0169560. [Google Scholar] [CrossRef] [PubMed]
- Zarmouh, N.O.; Mazzio, E.A.; Elshami, F.M.; Messeha, S.S.; Eyunni, S.V.; Soliman, K.F. Evaluation of the inhibitory effects of bavachinin and bavachin on human monoamine oxidases A and B. Evid. Based Complement. Alternat. Med. 2015, 852194. [Google Scholar] [CrossRef] [PubMed]
- Somani, G.; Kulkarni, C.; Shinde, P.; Shelke, R.; Laddha, K.; Sathaye, S. In vitro acetylcholinesterase inhibition by psoralen using molecular docking and enzymatic studies. J. Pharm. Bioallied Sci. 2015, 7, 32–36. [Google Scholar] [PubMed]
- Choi, Y.H.; Yon, G.H.; Hong, K.S.; Yoo, D.S.; Choi, C.W.; Park, W.K.; Kong, J.Y.; Kim, Y.S.; Ryu, S.Y. In vitro BACE-1 inhibitory phenolic components from the seeds of Psoralea corylifolia. Planta Med. 2008, 74, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Yang, Y.F.; Zhang, Y.T.; Yang, D.H. Dietary total prenylflavonoids from the fruits of Psoralea corylifolia L. prevents age-related cognitive deficits and down-regulates Alzheimer’s markers in SAMP8 mice. Molecules 2018, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zheng, X.W.; Qin, G.W.; Gai, Y.; Jiang, Z.H.; Guo, L.H. In vitro dopaminergic neuroprotective and in vivo antiparkinsonian-like effects of delta 3,2-hydroxybakuchiol isolated from Psoralea corylifolia (L.). Cell. Mol. Life Sci. 2009, 66, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Yang, Y.F.; Zhang, Y.T. Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro. FEBS Lett. 2013, 587, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Zhang, Y.B.; Chen, Z.J.; Zhang, Y.T.; Yang, X.W. Plasma pharmacokinetics and cerebral nuclei distribution of major constituents of Psoraleae Fructus in rats after oral administration. Phytomedicine 2018, 38, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Yun, B.R.; Kim, M.H.; Park, C.S.; Lee, W.S.; Oh, H.M.; Rho, M.C. Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation. Planta Med. 2001, 67, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.Y.; Ryu, J.H. Prenylflavones from Psoralea corylifolia inhibit nitric oxide synthase expression through the inhibition of I-κB-α degradation in activated microglial cells. Biol. Pharm. Bull. 2005, 28, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Fan, C.Q.; Wang, Y.; Dong, L.; Yue, J.M. Antibacterial prenylflavone derivatives from Psoralea corylifolia, and their structure–activity relationship study. Bioorg. Med. Chem. 2004, 12, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.Y.; Zhang, D.W.; Chen, R.D.; Yin, Y.Z.; Zou, J.H.; Xie, D.; Yang, L.; Wang, C.M.; Dai, J.G. Chemical constituents from cell cultures of Morus alba. China J. Chin. Mater. Med. 2012, 37, 3738–3742. [Google Scholar]
- Pistelli, L.; Spera, K.; Flamini, G.; Mele, S.; Morelli, I. Isoflavonoids and chalcones from Anthyllis hermanniae. Phytochemistry 1996, 42, 1455–1458. [Google Scholar] [CrossRef]
- Kraft, A.D.; Harry, G.J. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health 2011, 8, 2980–3018. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, V.; Thellung, S.; Corsaro, A.; Novelli, F.; Tasso, B.; Colucci-D’Amato, L.; Gatta, E.; Tonelli, M.; Florio, T. Celecoxib Inhibits Prion Protein 90-231-Mediated Pro-inflammatory Responses in Microglial Cells. Mol. Neurobiol. 2016, 53, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Rossetti, C. Neurodegenerative diseases: The immunological perspective. J. Neuroimmunol. 2017, 313, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Hemmens, B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem. Sci. 1997, 22, 477–481. [Google Scholar] [CrossRef]
- Villa, V.; Thellung, S.; Bajetto, A.; Gatta, E.; Robello, M.; Novelli, F.; Tasso, B.; Tonelli, M.; Florio, T. Novel celecoxib analogues inhibit glial production of prostaglandin E2, nitric oxide, and oxygen radicals reverting the neuroinflammatory responses induced by misfolded prion protein fragment 90-231 or lipopolysaccharide. Pharmacol. Res. 2016, 113, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Yang, Y.F.; Yang, X.W. Blood-brain barrier permeability and neuroprotective effects of three main alkaloids from the fruits of Euodia rutaecarpa with MDCK-pHaMDR cell monolayer and PC12 cell line. Biomed. Pharmacother. 2018, 98, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Han, Q.H.; Yuan, Q.H.; Meng, X.L.; Huo, J.; Bao, Y.X.; Xie, G.H. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget 2017, 8, 42001–42006. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Nicoll, J.A.R.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Chang, W.N.; Tsai, N.W.; Huang, C.C.; Kung, C.T.; Su, Y.J.; Lin, W.C.; Cheng, B.C.; Su, C.M.; Chiang, Y.F.; et al. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: A systematic review. Biomed. Res. Int. 2014, 2014, 182303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An Update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sala Frigerio, C.; De Strooper, B. Alzheimer’s disease mechanisms and emerging roads to novel therapeutics. Annu. Rev. Neurosci. 2016, 39, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Mobashir, M.; Hoda, N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 107, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.; Wei, G.; Mousseau, N.; Derreumaux, P. Pathway complexity of Alzheimer’s β-amyloid Aβ16-22 peptide assembly. Structure 2004, 12, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Balbach, J.J.; Ishii, Y.; Antzutkin, O.N.; Leapman, R.D.; Rizzo, N.W.; Dyda, F.; Reed, J.; Tycko, R. Amyloid Fibril Formation by Aβ16-22, a Seven-Residue Fragment of the Alzheimer’s β-Amyloid Peptide, and Structural Characterization by Solid State NMR. Biochemistry 2000, 39, 1374–1375. [Google Scholar] [CrossRef]
- Pizzi, A.; Dichiarante, V.; Terraneo, G.; Metrangolo, P. Crystallographic insights into the self-assembly of KLVFF amyloid-beta peptides. Biopolymers 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, X.; Yang, X.W. New sesquiterpenoids from the dried flower buds of Tussilago farfara and their inhibition on NO production in LPS-induced RAW264.7 cells. Fitoterapia 2012, 83, 318–322. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are not available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.-X.; Hu, Y.; Li, G.-Y.; Xu, W.; Zhang, Y.-T.; Yang, X.-W. Multi-Target Anti-Alzheimer Activities of Four Prenylated Compounds from Psoralea Fructus. Molecules 2018, 23, 614. https://doi.org/10.3390/molecules23030614
Xu Q-X, Hu Y, Li G-Y, Xu W, Zhang Y-T, Yang X-W. Multi-Target Anti-Alzheimer Activities of Four Prenylated Compounds from Psoralea Fructus. Molecules. 2018; 23(3):614. https://doi.org/10.3390/molecules23030614
Chicago/Turabian StyleXu, Qing-Xia, Ying Hu, Gui-Yang Li, Wei Xu, Ying-Tao Zhang, and Xiu-Wei Yang. 2018. "Multi-Target Anti-Alzheimer Activities of Four Prenylated Compounds from Psoralea Fructus" Molecules 23, no. 3: 614. https://doi.org/10.3390/molecules23030614