Chalcogen ‘like-like’ Interactions Involving Trisulphide and Triselenide Compounds: A Combined CSD and Ab Initio Study
Abstract
:1. Introduction
2. Results and Discussion
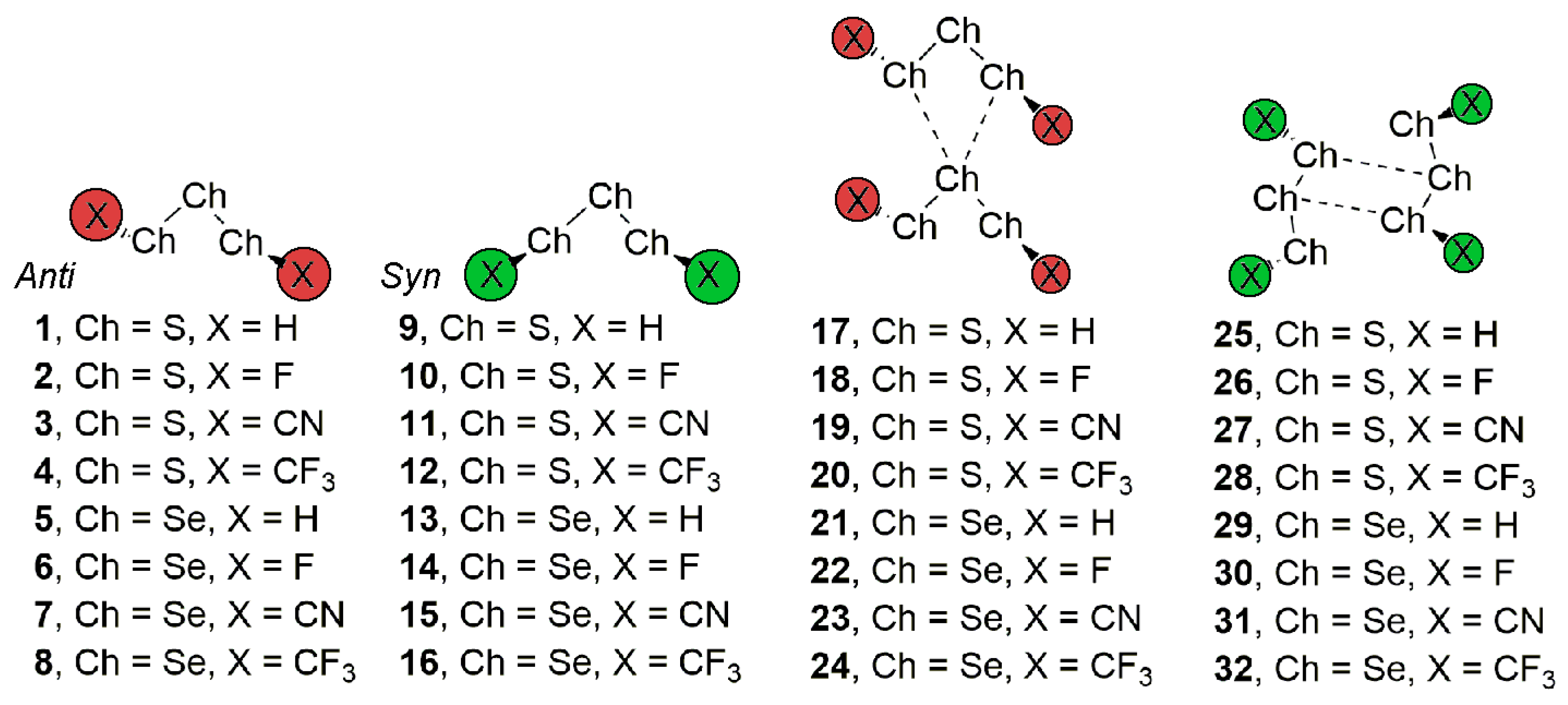
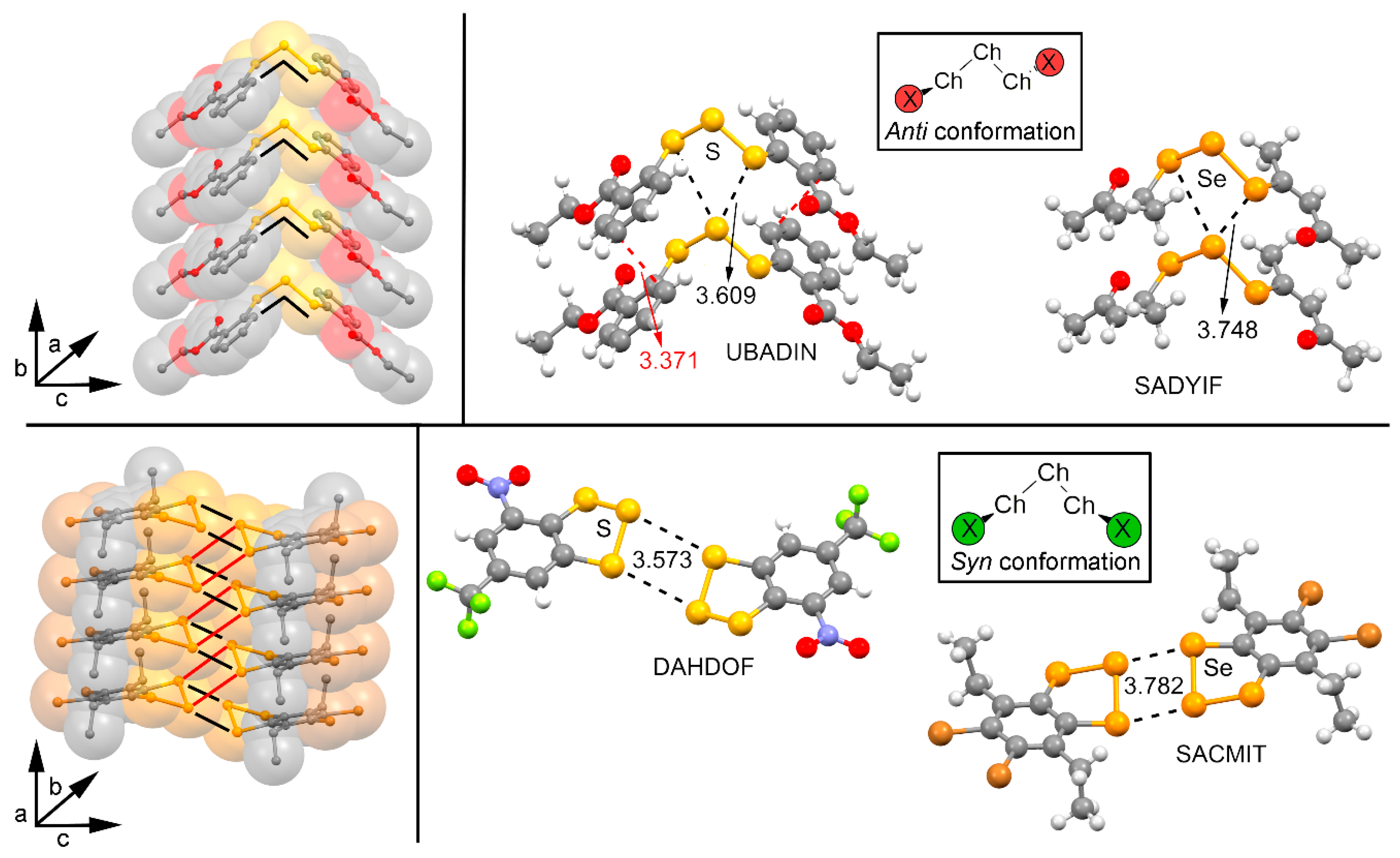
2.1. Cambridge Structural Database Search
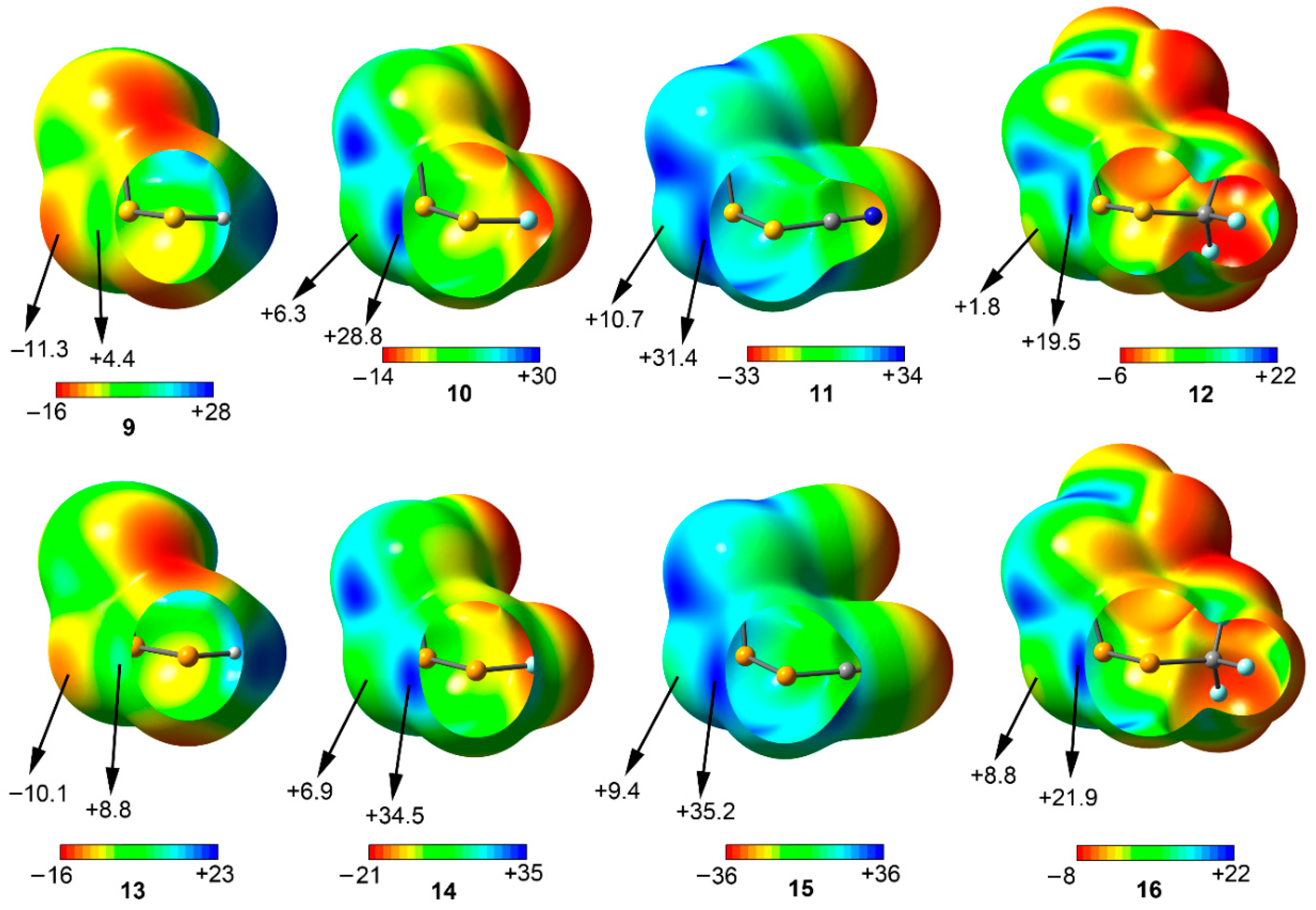
2.2. Preliminary MEP Analysis
2.3. Energetic and Geometric Results
2.4. “Atom in Molecules” and Natural Bond Order Analyses
3. Theoretical Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schneider, H.J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Yatsimirski, A. Principles and Methods in Supramolecular Chemistry; Wiley: Chichester, UK, 2000. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry Concepts and Perspectives; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Vögtle, F. Supramolecular Chemistry: An Introduction; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Beer, P.D.; Gale, P.A.; Smith, D.K. Supramolecular Chemistry; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Wiley: Chichester, UK, 2000. [Google Scholar]
- Grabowski, S.J. What is the covalency of hydrogen bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Murrayrust, P.; Motherwell, W.D.S. Computer retrieval and analysis of molecular geometry. 4. Intermolecular interactions. J. Am. Chem. Soc. 1979, 101, 4374–4376. [Google Scholar] [CrossRef]
- Bauzá, A.; Quiñonero, D.; Deyà, P.M.; Frontera, A. Halogen bonding versus chalcogen and pnicogen bonding: A combined Cambridge structural database and theoretical study. CrystEngComm 2013, 15, 3137–3144. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional σ/π-Hole Interactions. ChemPhysChem 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Aerogen Bonding Interaction: A New Supramolecular Force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene. Molecules 2015, 20, 11297–11316. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Triel bonds-complexes of boron and aluminum trihalides and trihydrides with benzene. Struct. Chem. 2017, 28, 1163–1171. [Google Scholar] [CrossRef]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent Bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in Tetrel Bonds. J. Phys. Chem. A 2016, 120, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen Bond: A Sister Noncovalent Bond to Halogen Bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. π-Hole aerogen bonding interactions. Phys. Chem. Chem. Phys. 2015, 17, 24748–24753. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Ward, M.D. Versatile and Resilient Hydrogen-Bonded Host Frameworks. Acc. Chem. Res. 2016, 49, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Azofra, L.M.; Alkorta, I.; Scheiner, S. Noncovalent interactions in dimers and trimers of SO3 and CO. Theor. Chem. Acc. 2014, 133, 1586. [Google Scholar] [CrossRef]
- Azofra, L.M.; Alkorta, I.; Scheiner, S. Strongly bound noncovalent (SO3)n:H2CO complexes (n = 1, 2). Phys. Chem. Chem. Phys. 2014, 16, 18974–18981. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Alkorta, I.; Elguero, J. Characterizing Complexes with Pnicogen Bonds Involving sp2 Hybridized Phosphorus Atoms: (H2C=PX)2 with X = F, Cl, OH, CN, NC, CCH, H, CH3, and BH2. J. Phys. Chem. A 2013, 117, 6893–6903. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.J.; Ling, K.B.; Cockroft, S.L. The Origin of Chalcogen-Bonding Interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef] [PubMed]
- Wonner, P.; Vogel, L.; Kniep, F.; Huber, S.M. Catalytic Carbon-Chlorine Bond Activation by Selenium-Based Chalcogen Bond Donors. Chem. Eur. J. 2017, 23, 16972–16975. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; Macchione, M.; Verolet, Q.; Mareda, J.; Sakai, N.; Matile, S. Anion Transport with Chalcogen Bonds. J. Am. Chem. Soc. 2016, 138, 9093–9096. [Google Scholar] [CrossRef] [PubMed]
- Kusamoto, T.; Yamamoto, H.M.; Kato, R. Utilization of σ-Holes on Sulfur and Halogen Atoms for Supramolecular Cation···Anion Interactions in Bilayer Ni(dmit)2 Anion Radical Salts. Cryst. Growth Des. 2013, 13, 4533–4541. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums: The Lore and the Science; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Hye-Kyung, N.; Eun-Hee, K.; Min-Ah, C.; Jong-Min, P.; Do-Hee, K.; Young-Joon, S. Diallyl Trisulfide Induces Apoptosis in Human Breast Cancer Cells Through ROS-Mediated Activation of JNK and AP-1. Biochem. Pharmacol. 2012, 84, 1241–1250. [Google Scholar]
- Ixao, D.; Singh, S. Diallyl Trisulfide, a Constituent of Processed Garlic, Inactivates Akt to Trigger Mitochondrial Translocation of BAD and Caspase-Mediated Apoptosis in Human Prostate Cancer Cells. Carcinogenesis 2006, 27, 533–540. [Google Scholar]
- Benavides, G.; Squadrito, G.; Mills, R.; Patel, H.; Isbell, T.; Patel, R.; Darley-Usmar, V.; Doeller, J.; Kraus, D. Hydrogen Sulfide Mediates the Vasoactivity of Garlic. Proc. Nat. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hosono, T.; Hosono-Fukao, T.; Inada, K.; Tanaka, R.; Ogihara, J.; Ariga, T. Anticancer Effects of Diallyl Trisulfide Derived from Garlic. Asia Pac. J. Clin. Nutr. 2008, 1, 249–252. [Google Scholar]
- Vandenberg, J.; Perry, M.; Perrin, M.; Mann, S.; Ke, Y.; Hill, A. hERG K+ Channels: Structure, Function, and Clinical Significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Boukebbous, K.; Laifa, E.A.; De Mallmann, A.; Taoufik, M. Diethyl 2,2′-(trisulfane-1,3-diyl)dibenzoate. IUCrData 2016, 1, x161708. [Google Scholar] [CrossRef]
- Arnold, A.P.; Cavell, K.J.; Edwards, A.J.; Hoskins, B.F.; Peacock, E.J. Preparation and X-Ray Crystal-Structure of 4,4′-Triselenobis(pent-3-en-2-one). Aust. J. Chem. 1988, 41, 1601–1606. [Google Scholar] [CrossRef]
- Kimura, T.; Yomogita, A.; Matsutani, T.; Suzuki, T.; Tanaka, I.; Kawai, Y.; Takaguchi, Y.; Wakahara, T.; Akasaka, T. Preparation of phthalocyanines with eight benzylchalcogeno substituents from 5,6-dibromo-4,7-diethylbenzo[1,2,3]trichalcogenoles. J. Org. Chem. 2004, 69, 4716–4723. [Google Scholar] [CrossRef] [PubMed]
- Chenard, B.L.; Harlow, R.L.; Johnson, A.L.; Vladuchick, S.A. Synthesis, structure, and properties of pentathiepins. J. Am. Chem. Soc. 1985, 187, 3871–3879. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bauzá, A.; Alkorta, I.; Frontera, A.; Elguero, J. On the Reliability of Pure and Hybrid DFT Methods for the Evaluation of Halogen, Chalcogen, and Pnicogen Bonds Involving Anionic and Neutral Electron Donors. J. Chem. Theory Comput. 2013, 9, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bär, M.; Hacer, M.; Horn, H.; Kömel, C. Electronic structure calculations on workstation computers: The program system Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Boys, S.B.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll (Version 13.05.06); TK Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Sample Availability: not available. |

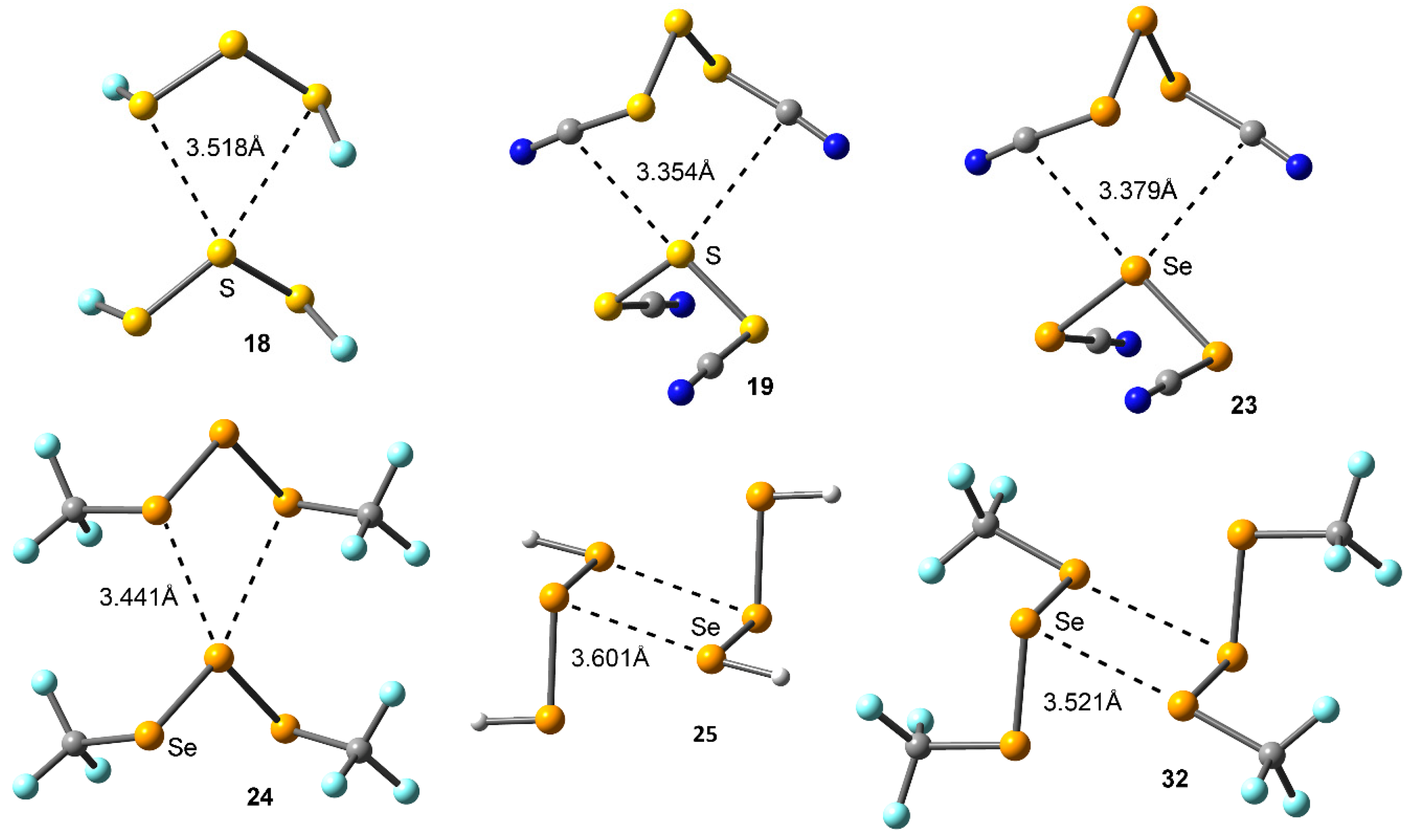
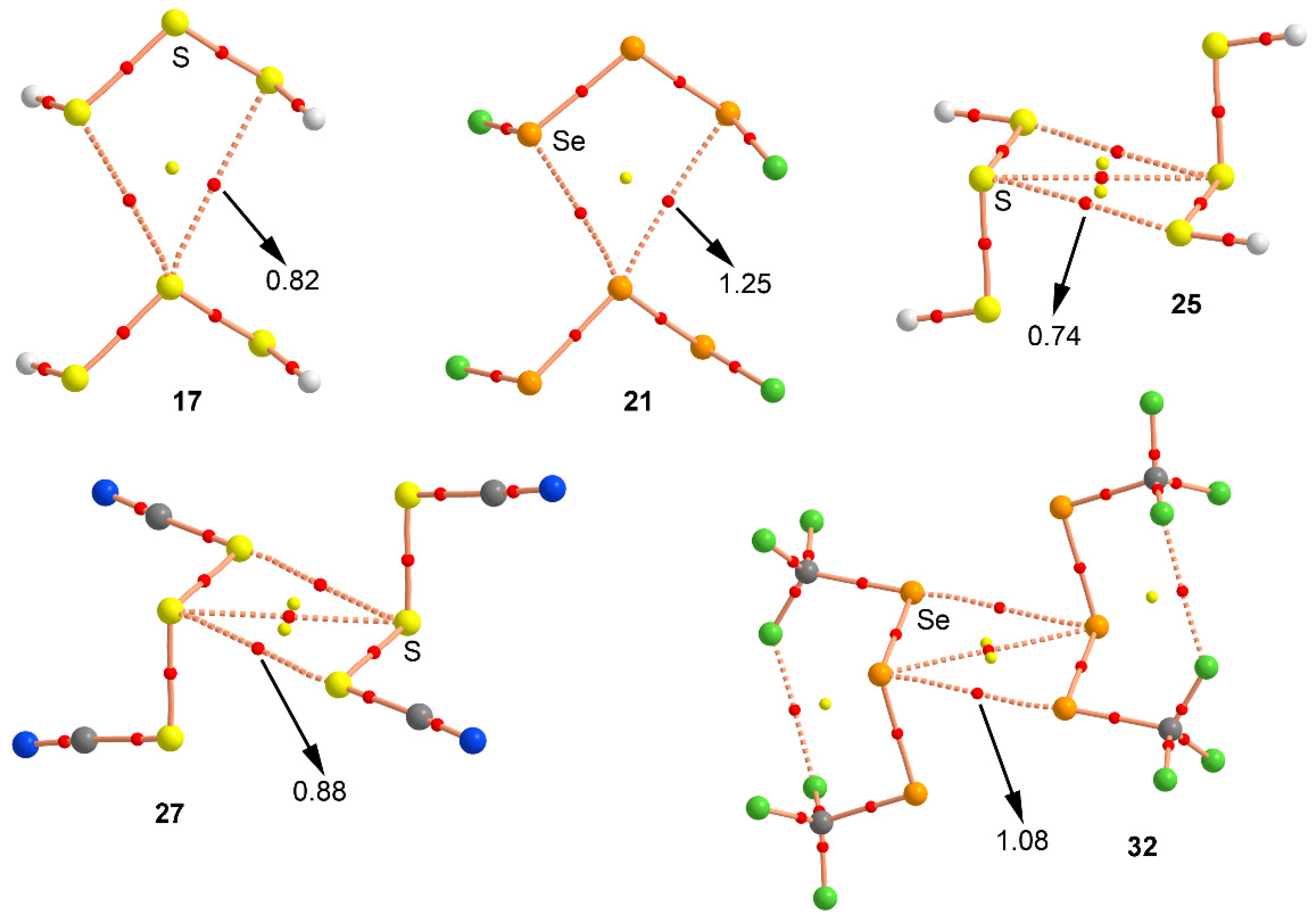
| Complex | ΔEBSSE | R | 102 x ρ | Nimag |
|---|---|---|---|---|
| 17 | −2.1 | 3.546 | 0.82 | 0 |
| 18 | −2.3 | 3.518 | 0.84 | 0 |
| 19 | −4.6 | 3.577 | 0.73 | 0 |
| 20 | −3.7 | 3.354 | 0.77 | 0 |
| 21 | −3.7 | 3.419 | 1.38 | 0 |
| 22 | −3.0 | 3.479 | 1.25 | 0 |
| 23 | −5.5 | 3.441 | 0.83 | 0 |
| 24 | −4.9 | 3.342 | 1.34 | 0 |
| 25 | −3.7 | 3.546 | 0.74 | 0 |
| 26 | −3.6 | 3.278 | 1.17 | 1 |
| 27 | −3.0 | 3.482 | 0.88 | 1 |
| 28 | −4.4 | 3.452 | 0.83 | 0 |
| 29 | −5.0 | 3.601 | 0.93 | 1 |
| 30 | −7.1 | 2.996 | 2.76 | 0 |
| 31 | −4.3 | 3.521 | 1.20 | 1 |
| 32 | −5.8 | 3.457 | 1.08 | 1 |
| Complex | Donor a | Acceptor | E(2) |
|---|---|---|---|
| 17 | LP S | BD* S–S | 1.12 |
| 18 | LP S | BD* S–S | 1.08 |
| 19 | BD C–N | BD* S–S | 0.84 |
| LP Se | BD* S–S | 0.22 | |
| 20 | LP S | BD* S–S | 0.94 |
| 21 | LP Se | BD* Se–Se | 3.62 |
| 22 | LP Se | BD* Se–Se | 2.70 |
| 23 | BD C–N | BD* Se–Se | 1.44 |
| LP Se | BD* Se–Se | 0.60 | |
| 24 | LP Se | BD* Se–Se | 3.46 |
| 25 | LP S | BD* S–H | 0.36 |
| 26 | LP S | BD* S–F | 1.08 |
| 27 | LP S | BD* S–C | 0.54 |
| 28 | LP S | BD* S–C | 0.36 |
| 29 | LP Se | BD* Se–H | 0.78 |
| 30 | LP Se | BD* Se–F | 5.40 |
| 31 | LP Se | BD* Se–C | 1.44 |
| 32 | LP Se | BD* Se–C | 1.28 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauzá, A.; Frontera, A. Chalcogen ‘like-like’ Interactions Involving Trisulphide and Triselenide Compounds: A Combined CSD and Ab Initio Study. Molecules 2018, 23, 699. https://doi.org/10.3390/molecules23030699
Bauzá A, Frontera A. Chalcogen ‘like-like’ Interactions Involving Trisulphide and Triselenide Compounds: A Combined CSD and Ab Initio Study. Molecules. 2018; 23(3):699. https://doi.org/10.3390/molecules23030699
Chicago/Turabian StyleBauzá, Antonio, and Antonio Frontera. 2018. "Chalcogen ‘like-like’ Interactions Involving Trisulphide and Triselenide Compounds: A Combined CSD and Ab Initio Study" Molecules 23, no. 3: 699. https://doi.org/10.3390/molecules23030699







