3. Materials and Methods
3.1. General Methods
Optical rotations were measured at 20 °C on a Perkin Elmer (Waltham, MA, USA) 341 polarimeter at a wave length of 589 nm and a path length of 10 cm.
NMR spectra were recorded on a Varian (Palo Alto, CA, USA) INOVA 500 operating at 499.82 MHz (
1H), and at 125.894 MHz (
13C), or on a Bruker (Billerica, MA, USA) Ultrashield spectrometer at 300.36 and 75.53 MHz, respectively. CDCl
3 was employed for protected compounds and CD
3OD as well as D
2O for unprotected inhibitors. Chemical shifts are listed in delta employing residual, non-deuterated solvent as the internal standard. Signals were assigned unambiguously by COSY and HSQC analysis. The signals of the
N-dansyl group are located in the expected regions and are not listed explicitly. For easier comparison with other
N-acetylgalactosaminide analogues, interpretation of NMR-spectra was performed according to the carbohydrate-related numbering system depicted in
Scheme 2. MALDI-TOF and EI-TOF mass spectrometry were performed on a Micromass (Waters Corporation, Milford, MA, USA) TofSpec 2E Time-of-Flight mass spectrometer. Analytical TLC was performed on pre-coated aluminum plates silica gel 60 F254 (E. Merck, Darmstadt, Germany 5554) and detected with UV light (254 nm). For staining, a solution of vanillin (9 g) in a mixture of H
2O (950 mL)/EtOH (750 mL)/H
2SO
4 (120 mL) or ceric ammonium molybdate (100 g ammonium molybdate/8 g ceric sulfate in 1 l 10% H
2SO
4) were employed, followed by heating on a hotplate. For column chromatography, silica gel 60 (230–400 mesh, E. Merck 9385) or silica gel 60 (Acros Organics (Thermo Fisher Scientific Inc., Waltham, MA, USA), AC 24036) were used. CCDC contains the
supplementary crystallographic data for this paper. These data can be obtained free of charge via
http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail:
[email protected]).
3.2. Biochemical Methods
Streptomyces plicatus N-acetyl-β-hexosaminidase, SpHex, was expressed and purified in
E. coli as described previously [
27]. Kinetic studies were performed at 25 °C in the assay buffer (sodium phosphate (50 mM), sodium citrate (50 mM), NaCl (100 mM), BSA (2 mg/mL), pH = 6.0). The enzyme was incubated with different concentrations of the inhibitors for 2–5 min prior to the start of the reaction by addition of the substrate (4-nitrophenyl
N-acetyl-β-
d-glucosaminide) and the initial rates were measured by monitoring the increase in absorbance at 405 nm for three to five minutes using a microplate reader (Synergy H1, BioTek, VT, USA).
Ki determinations were performed using two or three different substrate concentrations. For each one of these substrate concentrations a range of five to eight different inhibitor concentrations bracketing the ultimately determined
Ki value were used. Dixon plots (1/rate vs [I]) were constructed to validate the use of a competitive inhibition model. The data were then fit to a competitive inhibition model using non-linear regression analysis with GraFit 7.0 (Erithacus Software, UK). Assays were done twice using enzyme concentrations of 0.3 nM and 0.03 nM respectively in order to check compliance with the assumptions of Michaelis Menten kinetics ([E] << [I]). In addition, for compound
25, the assays were done a third time with the concentration of enzyme lowered to 0.003 nM. In all cases, the
Ki values were in good agreement.
Human skin fibroblasts (wild type) were grown in minimal essential medium (MEM) with Earle’s Salts (Sigma Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum, 400 µM l-glutamine, and 50 µg/mL gentamycin at 37 °C and 5% CO2. All cells used in this study were between the third and nineteenth passages.
All inhibitors were dissolved in DMSO in a concentration of 10 mM and diluted in 10 mM phosphate buffer (pH 7.0) containing 100 mM NaCl, 0.01% NaN3, and 0.01% Triton for the IC50-measurements.
Human N-acetyl-β-hexosaminidase A activity measurements were performed in duplicate assays unless otherwise stated. Fibroblast cells of three single 80 cm2 flasks were harvested by trypsinization in 500 µL (each of them) 0.9% NaCl containing 0.01% Triton, homogenized by sonication (4 times 15 s, Bandelin (Berlin, Germany) Sonopuls ultrasound homogenator mini 20) and centrifuged at 13.000 rpm for 1 min in a table top centrifuge (Biofuge Pico, Heraeus, Hanau, Germany). Protein amounts were determined according to the method of Lowry. For assessment of N-acetyl-β-hexosaminidase A activity, 10 µL (diluted 1:10) of cell homogenate were mixed with 90 µL 0.9% NaCl and 200 µL of 1 mM (4-methyl)umbelliferyl N-acetyl-β-glucosaminide-6-sulfate. Na (Glycosynth, Warrington, UK) in McIlvains phosphate/citrate puffer, pH 4,4. After incubation at 37 °C for 60 min, the reaction was stopped by adding 2.5 mL 400 mM glycine/NaOH (pH 10.4). The amount of hydrolyzed 4-methylumbelliferone was determined with a fluorescence spectrometer (F7000 Hitachi, Chiyoda, Japan).
Modified β-hexosaminidase A assays were used to estimate the half maximal inhibitory concentration (IC50) of the particular inhibitor. For IC50 determination, 0.001 to 100 µM of inhibitor was added to the assay mixture.
Activity was measured in normal fibroblasts. Data analysis was performed with Microcal™ Origin® v6.0 (Origin Lab, Northampton, MA, USA) using a non-linear curve fitting module based on sigmoid curve fitting.
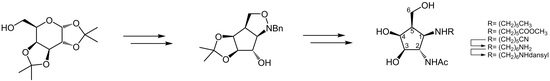
3.3. (3aR,3bS,6aR,7R,7aR)-Hexahydro-5,5-dimethyl-1-phenyl-1H-[1,3]dioxolo[3,4]cyclopent[1,2-c]isoxazol-7-ol or 1-l-(1,2,3,4,5)-11,21-Anhydro-1-hydroxymethyl-2-(N-hydroxy)benzylamino-4,5-O-isopropylidene-3,4,5-cyclopentanetriol 14
(a) Via Swern oxidation: To a solution of oxalyl chloride (1.12 mL, 13.0 mmol) in CH2Cl2 (20 mL), DMSO (1.11 mL, 15.7 mmol) was added dropwise at −60 °C. After 15 min, a 50% solution (w/v) of alcohol 12 (1.52 g, 5.22 mmol) in CH2Cl2 was added and the reaction was stirred for 15 min when Et3N (2.89 mL, 20.9 mmol) was added. The reaction mixture was allowed to reach ambient temperature and methanol (50 mL) and NaBH4 (0.39 g, 10.4 mmol) were added. When completed conversion of cyclopentanone 13 was observed (tlc, 30 min), solvents were removed under reduced pressure and crude alcohol 14 was dissolved in CH2Cl2. The organic layer was extracted with saturated aqueous NaHCO3, dried (Na2SO4), filtered and concentrated under reduced pressure. Purification on silica gel (cyclohexane/ethyl acetate 3:1 v/v) provided compound 14 as a pale yellow syrup (848 mg, 2.91 mmol, 55.8% from epimer 12).
(b) Via Dess-Martin oxidation: A 10% solution (w/v) of alcohol 12 (1.05 g, 3.60 mmol) in CH2Cl2 was stirred with Dess-Martin periodinane (1.68 g, 3.96 mmol) at ambient temperature for 10 min. After completed conversion, the reaction mixture was washed with saturated aqueous NaHCO3, dried (Na2SO4), and filtered. Removal of solvents under reduced pressure gave the crude product 13.
To a solution of crude ketone 13 in methanol (20 mL), NaBH4 (0.273 g, 7.21 mmol) was added. When completed conversion was detected (tlc, 30 min), solvents were removed under reduced pressure, and the crude product was dissolved in CH2Cl2. The organic layer was extracted with saturated aqueous NaHCO3, dried (Na2SO4), filtered, and concentrated under reduced pressure. Purification on silica gel (cyclohexane/ethyl acetate 3:1 v/v) provided compound 14 as a pale yellow syrup (0.769 g, 2.64 mmol, 73.2% over two steps).
After extended storage, a compound sample provided a few minute crystals, one of which could be exploited for X-ray structure determination (CCDC 1826202).
: +44.6 (c = 0.86, CHCl3); 1H-NMR (300 MHz, CDCl3) δ = 7.42–7.23 (m, 5H, aromatic NBn), 4.58 (dd, 1H, J2,3 = J3,4 = 5.5 Hz, H-3), 4.50 (dd, 1H, J4,5 = 7.5 Hz, H-4), 4.33 (d, 1H, J5,6a < 1 Hz, J6a,6b = 8.7 Hz, H-6a) 4.12 (d, 1H, J = 13.3 Hz, N-CH2-Ph), 4.04 (m, 1H, H-2), 3.97 (dd, 1H, J5,6b = 6.4 Hz, H-6b), 3.91 (dd, 1H, N-CH2-Ph), 3.68 (dd, 1H, J1,2 = 7.6 Hz, H-1), 3.48 (bs, 1H, 6-OH), 3.07 (ddd, 1H, H-5), 1.57, 1.25 (2s, 3H each, C(CH3)2). 13C-NMR (75.5 MHz, CDCl3): δ = 137.2 (ipso NBn), 129.2, 128.6, 127.6 (aromatic NBn), 112.8 (C(CH3)2), 81.7 (C-3), 78.3 (C-4), 73.2 (C-1), 71.2 (C-2), 65.6 (C-6), 62.1 (N-CH2-Ph), 49.6 (C-5), 25.4, 25.0 (C(CH3)2).
MS (MALDI): Calcd for [C16H21NO4Na]: m/z 314.1368 [M + Na]+; Found [M + Na]+ 314.1368.
3.4. (3aR,3bS,6aR,7S,7aR)-Hexahydro-7-azido-5,5-dimethyl-1-phenyl-1H-[1,3]dioxolo[3,4]cyclopent[1,2-c]isoxazol or 1-l-(1,2,4,5/3)-11,21-Anhydro-3-azido-1-hydroxymethyl-2-(N-hydroxy)benzylamino-4,5-O-isopropylidene-4,5-cyclopentanediol 16
A solution of alcohol 14 (848 mg, 2.91 mmol) in CH2Cl2 (20 mL) was cooled to 0 °C. Pyridine (0.940 mL, 11.6 mmol) and trifluoromethanesulfonyl anhydride (0.637 mL, 3.78 mmol) were added. When completed conversion of the starting material was observed (10 min), the reaction mixture was washed consecutively with HCl (6%) and saturated aqueous NaHCO3. After drying with Na2SO4, the suspension was filtered, and the solvent was removed at room temperature under reduced pressure. Resulting crude triflate 15 was dissolved in DMF (20 mL), NaN3 (1.14 g, 17.5 mmol) was added and the mixture was stirred at ambient temperature for 60 min. The reaction mixture was then concentrated under reduced pressure, the residue was dissolved with CH2Cl2, and the solution was washed with brine. The organic layer was dried (Na2SO4), filtered, and concentrated under reduced pressure. Purification of the remaining residue on silica gel (cyclohexane/ethyl acetate 10:1 v/v) provided azidodeoxy compound 16 (568 mg, 1.80 mmol, 61.7% from 14). : +74.8 (c = 1.09, CHCl3); 1H-NMR (300 MHz, CDCl3) δ = 7.44–7.23 (m, 5H, aromatic NBn), 4.59 (dd, 1H, J3,4 = J4,5 = 6.9 Hz, H-4), 4.31 (dd, 1H, J5,6a = 3.5 Hz, J6a,6b = 8.9 Hz, H-6a), 4.28 (m, 1H, H-3), 4.07 (d, 1H, H-6b), 4.01 (d, 1H, J = 12.6 Hz, N-CH2-Ph), 3.78 (dd, 1H, J1,2 = J2,3 = 7.5 Hz, H-2), 3.70 (d, 1H, N-CH2-Ph), 3.61 (dd, 1H, J1,5 = 8.2 Hz, H-1), 3.29 (dddd, 1H, H-5), 1.54, 1.29 (2s, 3H each, C(CH3)2. 13C-NMR (75.5 MHz, CDCl3): δ = 136.5 (ipso NBn), 129.1, 128.6, 127.8 (aromatic NBn), 113.4 (C(CH3)2), 84.5 (C-3), 77.4 (C-4), 75.6 (C-1), 69.9 (C-2), 64.9 (C-6), 59.5 (N-CH2-Ph), 45.6 (C-5), 27.7, 25.7 (C(CH3)2).
MS (EI): Calcd for [C16H20N4O3]: m/z 316.1535 [M]+; Found [M]+ 316.1532.
3.5. (3aR,3bS,6aR,7S,7aR)-Hexahydro-7-acetamido-5,5-dimethyl-1-phenyl-1H-[1,3]dioxolo[3,4]cyclopent[1,2-c]isoxazol or 1-l-(1,2,4,5/3)-11,21-Anhydro-3-acetamido-1-hydroxymethyl-2-(N-hydroxy)benzylamino-4,5-O-isopropylidene-4,5-cyclopentanediol 18
To a stirred suspension of zinc (1.17 g, 18.0 mmol) and NH4Cl (0.961 g, 18.0 mmol) in methanol (20 mL) a 50% solution (w/v) of azidocyclopentane 16 (848 mg, 2.91 mmol) in methanol was added. After completed conversion of the starting material (2 h), the mixture was filtered and concentrated under reduced pressure. The resulting crude amine 17 was dissolved in pyridine (20 mmol) and treated with acetic anhydride (0,255 mL, 2.69 mmol) and 4-DMAP (5 mg) at 0 °C. After completed consumption of amine 17, the reaction was quenched with methanol, and the solvents were removed under reduced pressure. The residue was dissolved in CH2Cl2 and consecutively washed with HCl (6%) and saturated aqueous NaHCO3, dried (Na2SO4) and filtered. Purification over silica gel chromatography (cyclohexane/ethyl acetate 2:1 v/v) provided acetamide 18 (422 mg, 1.27 mmol, 70.7% from 16) as a pale yellow syrup. : +11.1 (c = 0.82, CHCl3); 1H-NMR (300 MHz, CDCl3) δ = 7.38–7.23 (m, 5H, aromatic NBn), 6.11 (d, 1H, NHCOCH3), 4.95 (dd, 1H, J2,3 = J3,4 = 6.3 Hz, H-3), 4.75 (dd, 1H, J4,5 = 7.1 Hz, H-4), 4.29 (dd, 1H, J5,6a = 4.1 Hz, J6a,6b = 8.7 Hz, H-6a), 4.22 (dd, 1H, J1,2 = J1,5 = 8.0 Hz, H-1), 4.06 (dd, 1H, J5,6b = 8.9 Hz, H-6b), 3.98 (d, 1H, J = 12.9 Hz, N-CH2-Ph), 3.67 (d, 1H, N-CH2-Ph), 3.42 (m, 1H, H-5), 3.34 (dd, 1H, H-2), 1.83 (s, 3H, NHCOCH3), 1.51, 1.29 (2s, 3H each, C(CH3)2). 13C-NMR (75.5 MHz, CDCl3): δ = 170.7 (NHCOCH3), 137.0 (ipso NBn), 129.2, 128.5, 127.6 (aromatic NBn), 112.6 (C(CH3)2), 83.2 (C-3), 78.4 (C-4), 74.3 (C-1), 65.4 (C-6), 63.9 (C-2), 59.9 (N-CH2-Ph), 47.1 (C-5), 27.3, 25.4 (C(CH3)2), 23.6 (NHCOCH3).
After extended storage, a compound sample provided small crystals which could be employed for X-ray structure determination (CCDC 1826203).
MS (EI): Calc for [C18H24N2O4]: m/z 332.1736 [M]+; Found [M]+ 332.1737.
3.6. (3aS,4R,5R,6S,6aR)-5-Amino-tetrahydro-6-acetamido-2,2-dimethyl-4H-cyclopenta-1,3-dioxole-4-methanol or 1-l-(1,2,4,5/3)-3-Acetamido-2-amino-1-hydroxymethyl-4,5-O-isopropylidene-4,5-cyclopentanediol 19
A 5% solution of acetamide 18 (422 mg, 1.27 mmol) in methanol was stirred with Pearlman’s catalyst (Pd(OH)2/C, 20%) under an atmosphere of H2 at ambient pressure. After completed conversion (1 hour), the catalyst was filtered off, the filtrate was concentrated under reduced pressure, and the residue was chromatographically purified (chloroform/methanol/NH4OH (25%) 14:1:0.01 v/v/v) to obtain intermediate 19 as a pale yellow syrup (253 mg, 1.04 mmol, 81.6%). +7.5 (c = 0.85, CHCl3); 1H-NMR (300 MHz, CDCl3) δ = 7.29 (d, 1H, NHCOCH3), 4.68 (dd, 1H, J3,4 = 6.0 Hz, J4,5 = 5.7 Hz, H-4), 4.47 (d, 1H, H-3), 4.05 (d, 1H, J1,2 = 6.5 Hz, J4,5 < 1 Hz, H-2), 3.90 (m, 2H, H-6a, H-6b), 3.25 (d, 1H, J1,5 < 1 Hz, H-1), 3.00 (bs, 3H, 6-OH, 1-NH2), 2.41 (m, 1H, H-5), 1.95 (s, 3H, NHCOCH3), 1.45, 1.25 (2s, 3H each, C(CH3)2). 13C-NMR (75.5 MHz, CDCl3): δ = 170.7 (NHCOCH3), 111.0 (C(CH3)2), 85.4 (C-3), 80.8 (C-4), 63.2 (C-2), 59.3 (C-1), 58.1 (C-6), 47.2 (C-5), 26.5, 23.2 (C(CH3)2), 23.1 (NHCOCH3).
MS (MALDI): Calcd for [C11H20N2O4H]: m/z 245.1501 [M + H]+; Found [M + H]+ 245.1506.
3.7. (1S,2R,3S,4R,5R)-3-Acetamido-4-amino-5-hydroxymethylcyclopentanetriol or “1-amino-2-acetamido-2-deoxy-β-d-galacto-cyclopentane” 20
A solution of compound 19 (34.8 mg, 0.142 mmol) in methanol (1 mL) was treated with HCl (12 M 100µL). After completed deprotection, the solvent was removed under reduced pressure, and the remaining residue was purified by silica gel chromatography (chloroform/methanol/NH4OH (25%) 8:4:1 v/v/v) to furnish aminopolyol 20 as the free base (22.4 mg, 0.110 mmol, 77.0%). Treatment with HClg in methanol in the presence of small amounts of ethyl acetate as co-solvent afforded the corresponding hydrochloride (20.HCl) as a white solid. +57.6 (c = 0.90, H2O) (hydrochloride); 1H-NMR (500 MHz, D2O) (free base): δ = 4.21 (dd, 1H, J3,4 = J4,5 = 3.7 Hz, H-4), 4.12 (dd, 1H, J1,2 = 5.7 Hz, J2,3 = 9.1 Hz, H-2), 4.03 (dd, 1H, J3,4 = 3.8 Hz, H-3), 3.96 (dd, 1H, J5,6 = 7.4 Hz, J6a,6b = 11.2 Hz, H-6a), 3.90 (dd, 1H, J5,6b = 7.7 Hz, H-6b), 3.44 (dd, 1H, J1,5 = 8.7 Hz, H-1), 2.48 (dddd, 1H, J4,5 = 3.9 Hz, H-5), 2.10 (s, 3H, NHCOCH3). 13C-NMR (125.9 MHz, D2O) (free base): δ = 174.9 (NHCOCH3), 75.7 (C-3), 72.2 (C-4), 62.0 (C-2), 57.0 (C-6), 55.6 (C-1), 42.9 (C-5), 21.9 (NHCOCH3).
MS (MALDI): Calcd for [C8H16N2O4H]: m/z 205.1188 [M + H]+; Found [M + H]+ 2051184.
3.8. (1S,2R,3S,4R,5R)-N-(1-Hexyl)-3-acetamido-4-amino-5-hydroxymethylcyclopentanetriol or “2-Acetamido-2-deoxy-1-(hexyl)amino-β-d-galacto-cyclopentane” 21
Amine 19 (32.2 mg, 0.132 mmol) was dissolved in DMF (1 mL) and treated with 1-bromohexane (22.1 µL, 0.158 mmol) in the presence of NaHCO3 (53.2 mg, 0.633 mmol) at 60 °C. After completed consumption of the starting material, the mixture was concentrated under reduced pressure. The residue was diluted with methanol and treated with HCl (100 µL, 12 M) and stirred for one hour. After evaporation of the solvents, the remaining precipiate was purified by chromatography on silica gel (chloroform/methanol/NH4OH (25%) 8:1:0.01 v/v/v) to yield N-hexyl carbacycle 21 (25.4 mg, 881 µmol, 66.8% over two steps). : +49.8 (c = 0.97, MeOH); 1H-NMR (500 MHz, CD3OD): δ = 4.16 (dd, 1H, J1,2 = 5.0 Hz, J2,3 = 7.7 Hz, H-2), 4.08 (dd, 1H, J3,4 = J4,5 = 4.1 Hz, H-4), 3.96 (dd, 1H, J5,6b = 7.4 Hz, J6a,6b = 11.2 Hz, H-6a), 3.91 (m, 2H, H-3, H-6b), 3.21 (dd, 1H, J1,5 = 8.2 Hz, H-1), 2.94 (m, 1H, H-1′a), 2.73 (m, 1H, H-1′b), 2.44 (dddd, 1H, H-5), 2.04 (s, 3H, NHCOCH3), 1.58 (m, 2H, H-2′), 1.47–1.33 (m, 6H, H-3′, H-4′, H-5′), 0.97 (t, 3H, H-6′). 13C-NMR (125.9 MHz, CD3OD): δ = 173.3 (NHCOCH3), 78.9 (C-3), 74.0 (C-4), 64.6 (C-1), 63.0 (C-2), 59.1 (C-6), 47.9 (C-1′), 45.6 (C-5), 32.7, 29.6, 27.8, 23.6 (C-2′, C-3′, C-4′, C-5′) 22.8 (NHCOCH3), 14.3 (C-6′).
MS (MALDI): Calcd for [C14H28N2O4H]: m/z 289.2127 [M + H]+; Found [M + H]+ 289.2126.
3.9. (1S,2R,3S,4R,5R)-N-(Methoxycarbonyl)pentyl-3-acetamido-4-amino-5-hydroxymethyl-cyclopentanetriol or “2-Acetamido-2-deoxy-1-(methoxycarbonylhexyl)amino-β-d-galacto-cyclopentane” 22
Amine 19 (25.7 mg, 0.105 mmol) was dissolved in DMF (1 mL) and NaHCO3 (42.4 mg, 0.505 mmol) followed by methyl 6-iodohexanoate (20.8 mg, 0.505 mmol) were added. The reaction mixture was heated to 60 °C until completed consumption of the starting material was observed (tlc). The mixture was then concentrated under reduced pressure, and MeOH was added to obtain a ca. 50% solution. This was added to a mixture of methanol (5 mL) and acetic chloride (100 µL) at 0 °C, and the mixture was stirred for 15 min. Removal of solvent in vacuo and followed by chromatography of the residue (chloroform/methanol/NH4OH (25%) 8:1:0.01 v/v/v) gave free methyl ester 22 (23.8 mg, 71.6 µmol, 68.1% over two steps) as a colourless syrup. : +41.0 (c = 0.99, MeOH); 1H-NMR (300 MHz, CD3OD): δ = 4.07 (dd, 1H, J1,2 = 5.0 Hz, J2,3 = 7.4 Hz, H-2), 4.02 (dd, 1H, J3,4 = J4,5 = 4.1 Hz, H-4), 3.97–3.80 (m, 3H, H-3, H-6), 3.66 (s, 3H, H-1″), 3.06 (dd, 1H, J1,5 = 8.1 Hz, H-1), 2.83 (m, 1H, H-1′a), 2.56 (m, 1H, H-1′b), 2.42 – 2.28 (m, 3H, H-5, H-5′), 1.98 (s, 3H, NHCOCH3), 1.72–1.28 (m, 6H, H-2′, H-3′, H-4′). 13C-NMR (75.5 MHz, CD3OD): δ = 175.8 (COOMe), 173.1 (NHCOCH3), 79.1 (C-3), 74.0 (C-4), 64.5 (C-1), 63.6 (C-2), 59.1 (C-6), 52.0 (C-1″), 47.9 (C-1′), 46.0 (C-5), 34.7, 29.9, 27.7, 25.8 (C-2′, C-3′, C-4′, C-5′) 22.8 (NHCOCH3).
MS (MALDI): Calcd for [C15H28N2O6H]: m/z 333.2026 [M + H]+; Found [M + H]+ 333.2024.
3.10. (1S,2R,3S,4R,5R)-N-(6-Amino)hexyl-3-acetamido-4-amino-5-hydroxymethyl-cyclopentanetriol or “2-Acetamido-2-deoxy-1-(6-aminohexyl)amino-β-d-galacto-cyclopentane” 24
Amine 19 (68.2 mg, 0.279 mmol) was dissolved in DMF (3 mL) and treated with 6-bromohexanoic nitrile (44.4 µL, 0.335 mmol) in the presence of NaHCO3 (93.8 mg, 1.34 mmol). The reaction mixture was heated to 60 °C until completed consumption of the starting material was observed. The solvents were removed under reduced pressure. The residue was diluted with methanol, 2 M HCl (100 µL) was added, and the mixture was stirred for one hour. After evaporation of the solvents, the residue was purified by column chromatography (chloroform/methanol/NH4OH (25%) 8:1:0.01 v/v/v) to yield nitrile 23 (30.9 mg, 0.102 mmol, 37.0% from compound 19), which was directly used in the next step. : +24.3 (c = 0.975, MeOH); 1H-NMR (300 MHz, CD3OD): δ = 4.32 (dd, 1H, J1,2 = 5.2 Hz, J2,3 = 7.2 Hz, H-2), 4.10–3.94 (m, 4H, H-3, H-4, H-6a, H-6b), 3.64 (dd, 1H, J1,5 = 7.8 Hz, H-1), 3.23 (m, 2H, H-1′), 2.61 (m, 1H, H-5), 2.51 (t, 2H, H-5′), 2.05 (s, 3H, NHCOCH3), 1.84–1.45 (m, 6H, H-2′, H-3′, H-4′). 13C-NMR (75.5 MHz, CDCl3): δ = 174.1 (NHCOCH3), 121.0 (CN), 77.8 (C-3), 73.8 (C-4), 64.5 (C-1), 60.3 (C-2), 58.5 (C-6), 48.0 (C-1′), 44.5 (C-5), 26.6, 26.0, 25.9, (C-2′, C-3′, C-4′) 22.8 (NHCOCH3), 17.2 (C-5′).
A 10% solution of nitrile 23 (30.9 mg, 0.102 mmol) in methanol was stirred with small amounts of Raney-Ni under an atmosphere of H2 at ambient temperature. After full conversion of the starting material (20 min), the catalyst was filtered off, and the filtrate was concentrated under reduced pressure. Chromatographic purification (chloroform/methanol/NH4OH (25%) 8:4:1 v/v/v) afforded amine 24 as pale yellow syrup (30.9 mg, 0.102 mmol, 77.4%). Treatment with HClg provided the corresponding dihydrochloride 24·HCl as a white solid. : +38.5 (c = 1.115, H2O) (hydrochloride); 1H-NMR (300 MHz, CD3OD) (free base): δ = 4.14–4.03 (m, 2H, H-2, H-4), 3.86 (dd, 1H, J2,3 = 8.5 Hz, J3,4 = 4.2 Hz, H-3), 3.81 (d, 2H, H-6), 3.08 (dd, 1H, J1,2 = 5.6 Hz, J1,5 = 8.9 Hz, H-1), 2.76–2.56 (m, 3H, H-6′a, H-6′b, H-1′a), 2.47–2.34 (m, 2H, H-5, H-1′b), 2.00 (s, 3H, NHCOCH3), 1.57–1.21 (m, 8H, H-2′, H-3′, H-4′, H-5′). 13C-NMR (75.5 MHz, D2O) (free base): δ = 173.2 (NHCOCH3), 77.1 (C-3), 72.0 (C-4), 61.5 (C-2), 60.8 (C-1), 57.4 (C-6), 46.5 (C-1′), 43.8 (C-5), 40.2, 30.3, 28.0, 26.2, 25.7 (C-2′, C-3′, C-4′, C-5′, C-6′) 22.2 (NHCOCH3).
MS (MALDI): Calcd for [C14H29N3O4H]: m/z 304.2236 [M + H]+; Found [M + H]+ 304.2234.
3.11. (1S,2R,3S,4R,5R)-N-(6-Dansylamino)hexyl-3-acetamido-4-amino-5-hydroxymethyl-cyclopentanetriol or “2-Acetamido-2-deoxy-1-(6-dansylaminohexyl)amino-β-d-galacto-cyclopentane” 25
A solution of amine 24 (23.2 mg, 61.6 µmol) in methanol (1mL) was treated with Et3N (38.5 µL, 277 mmol) and dansyl chloride (18.3 mg, 67.8 µmol). After completed conversion of the starting material (30 min), the solvent was removed under reduced pressure. Purification on silica gel (chloroform/methanol/NH4OH (25%) 8:1:0.01 v/v/v) provided compound 25 (16.1 mg, 48.6 µmol, 78.9%) as light yellow, fluorescent syrup. : +23.7 (c = 0.960, MeOH); 1H-NMR (300 MHz, CD3OD): δ = 4.07 (dd, 1H, J1,2 = 4.9 Hz, J2,3 = 7.6 Hz, H-2), 4.01 (dd, 1H, J3,4 = J4,5 = 4.0 Hz, H-4), 3.93–3.79 (m, 3H, H-3, H-6a, H-6b), 3.09 (dd, 1H, J1,5 = 8.1 Hz, H-1), 2.90–2.71 (m, 3H, H-1’a, H-6’a, H-6’b), 2.53 (m, 1H, H-1’b), 2.35 (ddd, 1H, J5,6a = J5,6b = 7.2 Hz), 1.97 (s, 3H, NHCOCH3), 1.40–1.05 (m, 8H, H-2′, H-3′, H-4′, H-5′). 13C-NMR (75.5 MHz, CD3OD): δ = 173.2 (NHCOCH3), 78.9 (C-3), 74.0 (C-4), 64.6 (C-1), 63.1 (C-2), 59.0 (C-6), 47.9 (C-1′), 45.6 (C-5), 43.7, 30.4, 29.5, 27.5, 27.2 (C-2′, C-3′, C-4′, C-5′, C-6′) 22.8 (NHCOCH3).
MS (MALDI): Calcd for [C26H40N4O6SH]: m/z 537.2747 [M+H]+; Found [M+H]+ 537.2750.