Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies
Abstract
:1. Introduction
2. Structure–Activity Relationship Studies Performed with Fungal Metabolites
2.1. Fungicides
2.2. Bactericides
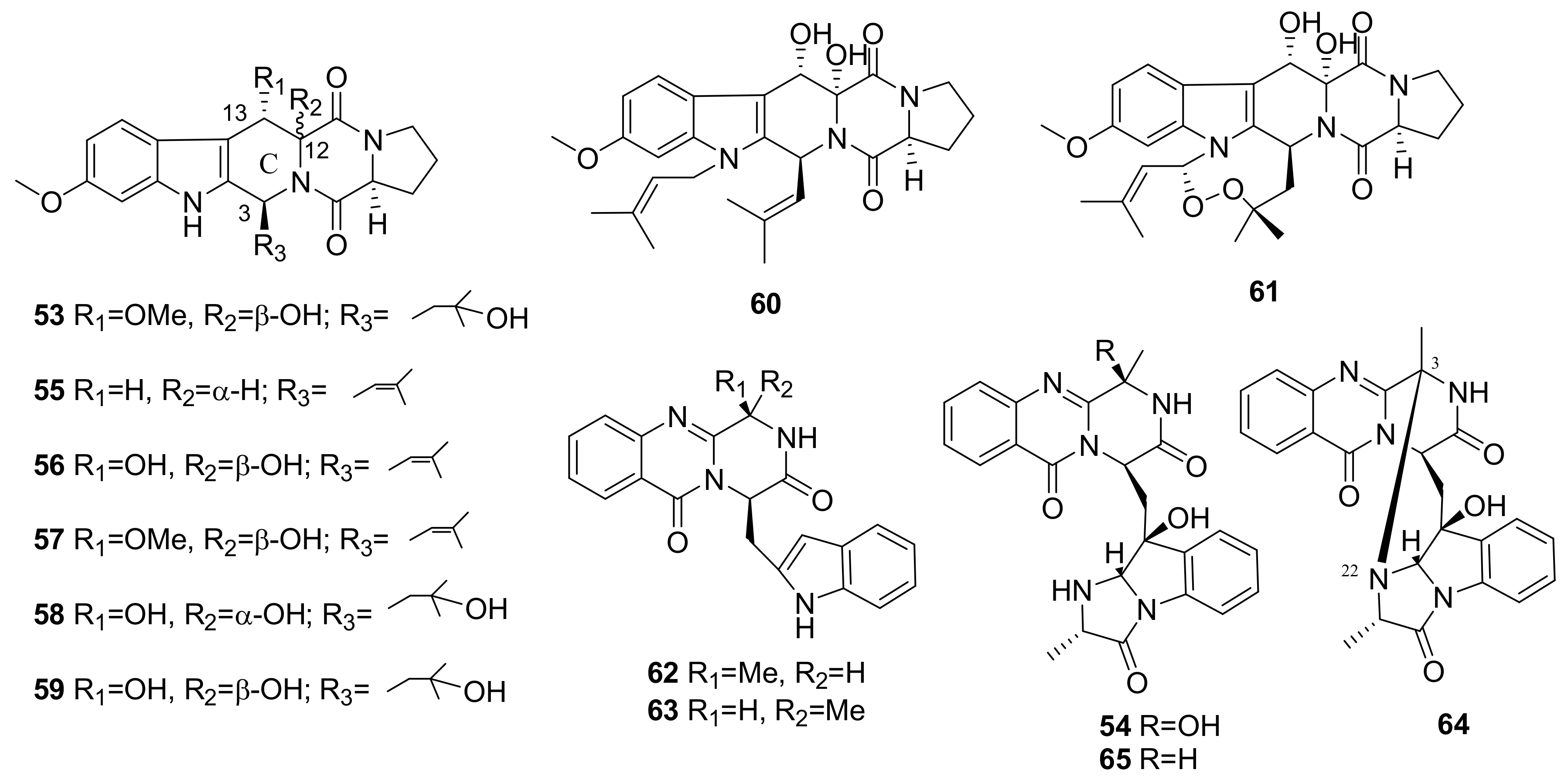
2.3. Insecticides
2.4. Herbicides
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ac2O | Acetic anhydride |
| at.p. | Atmospheric pressure |
| CH2N2 | Diazomethane |
| CH3CN | Acetonitrile |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAP | 4-Dimethylaminopyridine |
| EtOAc | Ethyl acetate |
| Et2O | Diethyl ether |
| MeOH | Methanol |
| Me2CO | Acetone |
| MIC | Minimum inhibitory concentration |
| PDB | Potato dextrose broth |
| p-TSA | p-Toluenesulfonic acid |
| rt | Room temperature |
| THF | Tetrahydrofuran |
References
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological control of mosquito vectors: Past, present, and future. Insects 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Finizio, A.; Villa, S. Environmental risk assessment for pesticides. Environ. Impact Assess. Rev. 2002, 22, 235–248. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, N.; Duraisamy, A. Ecological impact of pesticides principally organochlorine insecticide endosulfan: A review. Univ. J. Environ. Res. Technol. 2012, 2, 369–376. [Google Scholar]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. JOGNN J. Obstet. Gynecol. Neonatal. Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Bateman, D.N. Pesticides. Medicine 2012, 40, 147–150. [Google Scholar] [CrossRef]
- Pal, K.K.; Mc Spadden Gardener, B. Biological control of plant pathogens. Plant Health Instr. 2006, 2, 1117–1142. [Google Scholar] [CrossRef]
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Balog, A.; Hartel, T.; Loxdale, H.D.; Wilson, K. Differences in the progress of the biopesticide revolution between the EU and other major crop-growing regions. Pest Manag. Sci. 2017, 73, 2203–2208. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dikshit, A.K. Biopesticides: An ecofriendly approach for pest control. J. Biopestic. 2010, 3, 186–188. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Giedraitienė, A.; Vitkauskienė, A.; Naginienė, R.; Pavilonis, A. Antibiotic resistance mechanisms of clinically important bacteria. Medicina 2011, 47, 137–146. [Google Scholar] [PubMed]
- Taiwo, S.S. Antibiotic-resistant bugs in the 21st century: A public health challenge. World J. Clin. Infect. Dis. 2011, 1, 11–16. [Google Scholar] [CrossRef]
- Smith, K.F.; Guégan, J.F. Changing geographic distributions of human pathogens. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 231–250. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, R. Natural products as antifungal agents against clinically relevant pathogens. Nat. Prod. Rep. 2010, 27, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.; Evidente, A.; Vurro, M.; Mathieu, V.; Cimmino, A.; Evidente, M.; van Otterlo, W.A.L.; Dasari, R.; Lefranc, F.; Kiss, R. Toward a cancer drug of fungal origin. Med. Res. Rev. 2015, 35, 937–967. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; ISBN 9780470741689. [Google Scholar]
- Cole, R.J.; Cox, R.H. Handbook of Toxic Fungal Metabolites; Elsevier Inc.: Amsterdam, The Netherlands, 1981; ISBN 9780121797607. [Google Scholar]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Evidente, A.; Cimmino, A. Bioactive metabolites from pathogenic and endophytic fungi of forest trees. Curr. Med. Chem. 2018, 25, 208–252. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Masi, M.; Linaldeddu, B.T.; Franceschini, A.; Scanu, B.; Cimmino, A.; Andolfi, A.; Motta, A.; Maddau, L. Afritoxinones A and B, dihydrofuropyran-2-ones produced by Diplodia africana the causal agent of branch dieback on Juniperus phoenicea. Phytochemistry 2012, 77, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Maddau, L.; Basso, S.; Linaldeddu, B.T.; Cimmino, A.; Scanu, B.; Deidda, A.; Tuzi, A.; Evidente, A. Diplopimarane, a 20-nor-ent-pimarane produced by the oak pathogen Diplodia quercivora. J. Nat. Prod. 2014, 77, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Cimmino, A.; D’Amico, W.; Scanu, B.; Evidente, M.; Tuzi, A.; Evidente, A. Bioactive secondary metabolites produced by the oak pathogen Diplodia corticola. J. Agric. Food Chem. 2016, 64, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Maddau, L.; Masi, M.; Evidente, M.; Linaldeddu, B.T.; Evidente, A. Further secondary metabolites produced by Diplodia corticola, a fungal pathogen involved in cork oak decline. Tetrahedron 2016, 72, 6788–6793. [Google Scholar] [CrossRef]
- Lallemand, B.; Masi, M.; Maddau, L.; De Lorenzi, M.; Dam, R.; Cimmino, A.; Moreno, Y.; Banuls, L.; Andolfi, A.; Kiss, R.; et al. Evaluation of in vitro anticancer activity of sphaeropsidins A–C, fungal rearranged pimarane diterpenes, and semisynthetic derivatives. Phytochem. Lett. 2012, 5, 770–775. [Google Scholar] [CrossRef]
- Mathieu, V.; Chantôme, A.; Lefranc, F.; Cimmino, A.; Miklos, W.; Paulitschke, V.; Mohr, T.; Maddau, L.; Kornienko, A.; Berger, W.; et al. Sphaeropsidin A shows promising activity against drug-resistant cancer cells by targeting regulatory volume increase. Cell. Mol. Life Sci. 2015, 72, 3731–3746. [Google Scholar] [CrossRef] [PubMed]
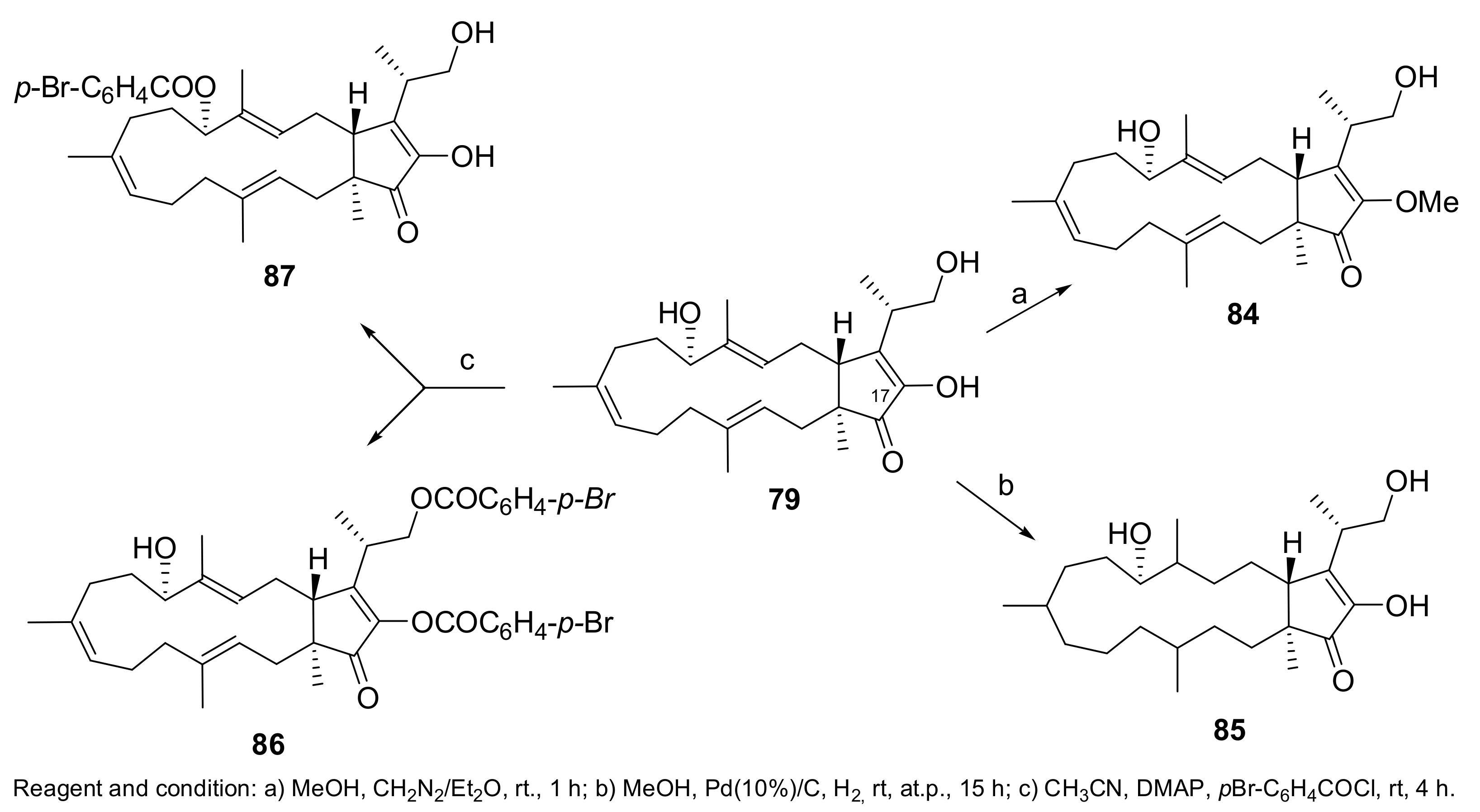
- Masi, M.; Cimmino, A.; Maddau, L.; Kornienko, A.; Tuzi, A.; Evidente, A. Crystal structure and absolute configuration of sphaeropsidin A and its 6-O-p-bromobenzoate. Tetrahedron Lett. 2016, 57, 4592–4594. [Google Scholar] [CrossRef]
- Ingels, A.; Dinhof, C.; Garg, A.D.; Maddau, L.; Masi, M.; Evidente, A.; Berger, W.; Dejaegher, B.; Mathieu, V. Computed determination of the in vitro optimal chemocombinations of sphaeropsidin A with chemotherapeutic agents to combat melanomas. Cancer Chemother. Pharmacol. 2017, 79, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Sparapano, L.; Motta, A.; Giordano, F.; Fierro, O.; Frisullo, S. A phytotoxic pimarane diterpene of Sphaeropsis sapinea f. sp. cupressi, the pathogen of a canker disease of cypress. Phytochemistry 1996, 42, 1541–1546. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Fierro, O.; Bruno, G.; Giordano, F.; Motta, A. Sphaeropsidins B and C, phytotoxic pimarane diterpenes from Sphaeropsis sapinea f. sp. cupressi and Diplodia mutila. Phytochemistry 1997, 45, 705–713. [Google Scholar] [CrossRef]
- Sparapano, L.; Bruno, G.; Fierro, O.; Evidente, A. Studies on structure-activity relationship of sphaeropsidins A–F, phytotoxins produced by Sphaeropsis sapinea f. sp. cupressi. Phytochemistry 2004, 65, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Maddau, L.; Scanu, B.; Andolfi, A.; Masi, M.; Motta, A.; Tuzi, A. Sphaeropsidones, phytotoxic dimedone methyl ethers produced by Diplodia cupressi: A structure—Activity relationship study. J. Nat. Prod. 2011, 74, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Cimmino, A.; Basso, S.; Evidente, A. Bioactivity studies of oxysporone and several derivatives. Phytochem. Lett. 2014, 10, 40–45. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Evidente, A.; Meyer, S.; Nicholson, J.; Munoz, A. Effect of strain and cultural conditions on the production of cytochalasin B by the potential mycoherbicide Pyrenophora semeniperda (Pleosporaceae, Pleosporales). Biocontrol. Sci. Technol. 2014, 24, 53–64. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Cimmino, A.; Clement, S.; Black, B.; Evidente, A. Pyrenophoric acids B and C, two new phytotoxic sesquiterpenoids produced by Pyrenophora semeniperda. J. Agric. Food Chem. 2014, 62, 10304–10311. [Google Scholar] [CrossRef] [PubMed]
- Skellam, E. The biosynthesis of cytochalasans. Nat. Prod. Rep. 2017, 34, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Capasso, R.; Evidente, A.; Randazzo, G.; Vurro, M. Cytochalasins: Structure-activity relationships. Phytochemistry 1990, 29, 93–96. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, Q.; Zhang, A.L.; Gao, J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Conti, L.; Altomare, C.; Bottalico, A.; Sindona, G.; Segre, A.L.; Logrieco, A. Fusapyrone and deoxyfusapyrone, two antifungal α-pyrones from Fusarium semitectum. Nat. Toxins 1994, 2, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Pengue, R.; Favilla, M.; Evidente, A.; Visconti, A. Structure-activity relationships of derivatives of fusapyrone, an antifungal metabolite of Fusarium semitectum. J. Agric. Food Chem. 2004, 52, 2997–3001. [Google Scholar] [CrossRef] [PubMed]
- Cooney, J.M.; Lauren, D.R. Trichoderma/pathogen interactions: Measurement of antagonistic chemicals produced at the antagonist/pathogen interface using a tubular bioassay. Lett. Appl. Microbiol. 1998, 27, 283–286. [Google Scholar] [CrossRef] [PubMed]
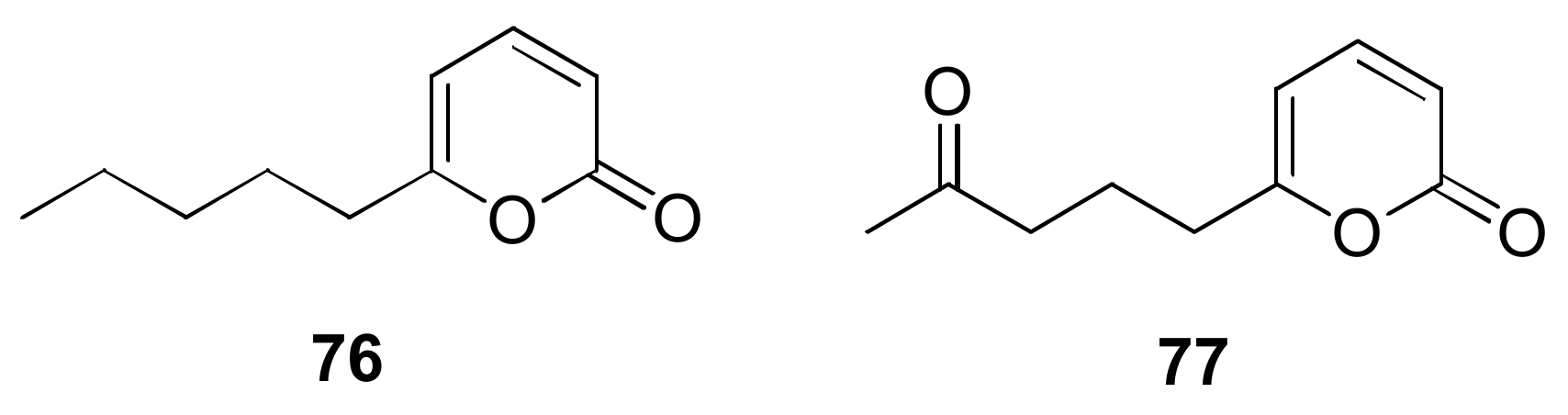
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-pentyl-2H-pyran-2-one (6-PAP) and other volatiles by different Trichoderma species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef]
- Evidente, A.; Cabras, A.; Maddau, L.; Serra, S.; Andolfi, A.; Motta, A. Viridepyronone, a New antifungal 6-substituted 2H-Pyran-2-one produced by Trichoderma viride. J. Agric. Food Chem. 2003, 51, 6957–6960. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S. Dung-inhabiting fungi: A potential reservoir of novel secondary metabolites for the control of plant pathogens. Pest Manag. Sci. 2016, 72, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Sarrocco, S.; Masi, M.; Diquattro, S.; Evidente, M.; Vannacci, G.; Evidente, A. Fusaproliferin, terpestacin and their derivatives display variable allelopathic activity against some Ascomycetous fungi. Chem. Biodivers. 2016, 13, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, X.F.; Zhou, M.G.; Kuang, J.; Zhang, Y.; Shang, Y.; Wang, J.X. Status of streptomycin resistance development in Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola in China and their resistance characters. J. Phytopathol. 2010, 158, 601–608. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Venturi, V.; Masi, M.; Degrassi, G.; Cimmino, A.; Maddau, L.; Andolfi, A. In vitro antibacterial activity of sphaeropsidins and chemical derivatives toward Xanthomonas oryzae pv. oryzae, the causal agent of rice bacterial blight. J. Nat. Prod. 2011, 74, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, Y.; Zhang, J.-R. Molecular basis of host specificity in human pathogenic bacteria. Emerg. Microbes Infect. 2014, 3, e23. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.L.; Le, X.; Li, H.J.; Yang, X.L.; Chen, J.X.; Xu, J.; Liu, H.L.; Wang, L.Y.; Wang, K.T.; Hu, K.C.; et al. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Andolfi, A.; Mathieu, V.; Boari, A.; Cimmino, A.; Moreno, Y.; Banuls, L.; Vurro, M.; Kornienko, A.; Kiss, R.; et al. Fischerindoline, a pyrroloindole sesquiterpenoid isolated from Neosartorya pseudofischeri, with in vitro growth inhibitory activity in human cancer cell lines. Tetrahedron 2013, 69, 7466–7470. [Google Scholar] [CrossRef]
- Schnekenburger, M.; Mathieu, V.; Lefranc, F.; Jang, J.Y.; Masi, M.; Kijjoa, A.; Evidente, A.; Kim, H.-J.; Kiss, R.; Dicato, M.; et al. The fungal metabolite eurochevalierine, a sequiterpene alkaloid, displays anti-cancer properties through selective sirtuin 1/2 inhibition. Molecules 2018, 23, 333. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, N.; Han, B.; Mei, Y.; Ge, H.; Guo, Z.; Weng, N.; Tan, R. Antibacterial epipolythiodioxopiperazine and unprecedented sesquiterpene from Pseudallescheria boydii, a beetle (coleoptera)-associated fungus. Org. Biomol. Chem. 2014, 12, 9405–9412. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Wei, M.Y.; Shao, C.L.; Fu, X.M.; Guo, Z.Y.; Xu, R.F.; Zheng, C.J.; She, Z.G.; Lin, Y.C.; Wang, C.Y. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J. Nat. Prod. 2012, 75, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. Spiromastixones A–O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus. J. Nat. Prod. 2014, 77, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, R.; Liu, M.; Liu, D.; Li, X.; Wang, S.; Niu, S.; Guo, P.; Lin, W.; Jacobson, P.B. Spiromastixones inhibit foam cell formation via regulation of cholesterol efflux and uptake in RAW264.7 macrophages. Mar. Drugs 2015, 13, 6352–6365. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Milatovic, D. Insecticides. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014; pp. 389–407. ISBN 9780124046306. [Google Scholar]
- Anke, H.; Sterner, O. Insecticidal and nematicidal metabolites from fungi. In The Mycota X. Industrial Applications; Osiewacz, H.D., Ed.; Springer: Berlin, Germany, 2002; pp. 109–127. [Google Scholar]
- Geris, R.; Ribeiro, P.R.; Da Silva Brandão, M.; Da Silva, H.H.G.; Da Silva, I.G. Chapter 10—Bioactive Natural Products as Potential Candidates to Control Aedes aegypti, the Vector of Dengue; Elsevier: New York, NY, USA, 2012; Volume 37. [Google Scholar]
- Masi, M.; Cala, A.; Tabanca, N.; Cimmino, A.; Green, I.R.; Bloomquist, J.R.; Van Otterlo, W.A.L.; Macias, F.A.; Evidente, A. Alkaloids with activity against the zika virus vector Aedes aegypti (L.)-crinsarnine and sarniensinol, two new crinine and mesembrine type alkaloids isolated from the South African plant Nerine sarniensis. Molecules 2016, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; van der Westhuyzen, A.E.; Tabanca, N.; Evidente, M.; Cimmino, A.; Green, I.R.; Bernier, U.R.; Becnel, J.J.; Bloomquist, J.R.; van Otterlo, W.A.L.; et al. Sarniensine, a mesembrine-type alkaloid isolated from Nerine sarniensis, an indigenous South African Amaryllidaceae, with larvicidal and adulticidal activities against Aedes aegypti. Fitoterapia 2017, 116, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Cimmino, A.; Tabanca, N.; Becnel, J.J.; Bloomquist, J.R.; Evidente, A. A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus. Open Chem. 2017, 15, 156–166. [Google Scholar] [CrossRef]
- Cimmino, A.; Andolfi, A.; Avolio, F.; Ali, A.; Tabanca, N.; Khan, I.A.; Evidente, A. Cyclopaldic acid, seiridin, and sphaeropsidin A as fungal phytotoxins, and larvicidal and biting deterrents against Aedes aegypti (Diptera: Culicidae): Structure-activity relationships. Chem. Biodivers. 2013, 10, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Evidente, M.; Masi, M.; Ali, A.; Tabanca, N.; Khan, I.A.; Evidente, A. Papyracillic acid and its derivatives as biting deterrents against Aedes aegypti (Diptera: Culicidae): Structure-activity relationships. Med. Chem. Res. 2015, 24, 3981–3989. [Google Scholar] [CrossRef]
- Graniti, A.; Sparapano, L.; Evidente, A. Cyclopaldic acid, a major phytotoxic metabolite of Seiridium cupressi, the pathogen of a canker disease of cypress. Plant Pathol. 2007, 41, 563–568. [Google Scholar] [CrossRef]
- Sparapano, L.; Evidente, A. Biological activity of cyclopaldic acid, a major toxin of Seiridium cupressi, its six derivatives, andiso-cyclopaldic acid. Nat. Toxins 1995, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Randazzo, G.; Ballio, A. Structure determination of seiridin and isoseiridin, phytotoxic butenolides from culture filtrate of Seiridium cardinale. J. Nat. Prod. 1986, 49, 593–601. [Google Scholar] [CrossRef]
- Shan, R.; Anke, H.; Stadler, M.; Sterner, O. Papyracillic acid, a new penicillic acid analogue from the ascomycete Lachnum papyraceum. Tetrahedron 1996, 52, 10249–10254. [Google Scholar] [CrossRef]
- Evidente, A.; Berestetskiy, A.; Cimmino, A.; Tuzi, A.; Superchi, S.; Melck, D.; Andolfi, A. Papyracillic acid, a phytotoxic 1,6-dioxaspiro[4,4]nonene produced by Ascochyta agropyrina var. nana, a potential mycoherbicide for Elytrigia repens biocontrol. J. Agric. Food Chem. 2009, 57, 11168–11173. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Stadler, M.; Anke, H.; Sterner, O. The reactivity of the fungal toxin papyracillic acid. Tetrahedron 1997, 53, 6209–6214. [Google Scholar] [CrossRef]
- Geris dos Santos, R.M.; Rodrigues-Fo, E. Meroterpenes from Penicillium sp. found in association with Melia azedarach. Phytochemistry 2002, 61, 907–912. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural diversity and biological activities of the cyclodipeptides from fungi. Molecules 2017, 22, 2026. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Furutani, S.; Otaka, J.; Noguchi, A.; Kinugasa, K.; Kai, K.; Hayashi, H.; Ihara, M.; Takahashi, S.; Matsuda, K.; et al. Biosynthesis and structure–activity relationship studies of okaramines that target insect glutamate-gated chloride channels. ACS Chem. Biol. 2018, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Takiuchi, K.; Murao, S.; Arai, M. Structure and insecticidal activity of new indole alkaloids, okaramines A and B, from Penicillium simplicissimum ak-40. Agric. Biol. Chem. 1989, 53, 461–469. [Google Scholar] [CrossRef]
- Harvey, R.J.; Vreugdenhill, E.; Zaman, S.H.; Bhandal, N.S.; Usherwood, P.N.R.; Barnard, E.A.; Darlison, M.G.; Harvey, R.J.; ffrench-Constant, R.H.; Rocheleau, T.A.; et al. GluCl a target of indole alkaloid okaramines: A 25 year enigma solved. Br. J. Pharmacol. 1996, 119, 62–67. [Google Scholar] [CrossRef]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Avolio, F.; Santini, A.; Tuzi, A.; Berestetskyi, A.; Vurro, M.; Evidente, A. Chenopodolin: A phytotoxic unrearranged ent-pimaradiene diterpene produced by phoma chenopodicola, a fungal pathogen for Chenopodium album biocontrol. J. Nat. Prod. 2013, 76, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Evidente, M.; Cimmino, A.; Zonno, M.C.; Masi, M.; Berestetskyi, A.; Santoro, E.; Superchi, S.; Vurro, M.; Evidente, A. Phytotoxins produced by Phoma chenopodiicola, a fungal pathogen of Chenopodium album. Phytochemistry 2015, 117, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Berestetskiy, A.; Dmitriev, A.; Mitina, G.; Lisker, I.; Andolfi, A.; Evidente, A. Nonenolides and cytochalasins with phytotoxic activity against Cirsium arvense and Sonchus arvensis: A structure-activity relationships study. Phytochemistry 2008, 69, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Cimmino, A.; Vurro, M.; Berestetskiy, A.; Troise, C.; Zonno, M.C.; Motta, A.; Evidente, A. Agropyrenol and agropyrenal, phytotoxins from Ascochyta agropyrina var. nana, a fungal pathogen of Elitrigia repens. Phytochemistry 2012, 79, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Zonno, M.C.; Andolfi, A.; Troise, C.; Motta, A.; Vurro, M.; Evidente, A. Agropyrenol, a phytotoxic fungal metabolite, and its derivatives: A structure-activity relationship study. J. Agric. Food Chem. 2013, 61, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Troise, C.; Santini, A.; Tuzi, A.; Vurro, M.; Ash, G.; Evidente, A. Phomentrioloxin: A phytotoxic pentasubstituted geranylcyclohexentriol produced by Phomopsis sp., a potential mycoherbicide for Carthamus lanatus biocontrol. J. Nat. Prod. 2012, 75, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Boari, A.; Troise, C.; Motta, A.; Vurro, M.; Ash, G.; Evidente, A. Phomentrioloxin, a fungal phytotoxin with potential herbicidal activity, and its derivatives: A structure-activity relationship study. J. Agric. Food Chem. 2013, 61, 9645–9649. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Fiore, M.; Boari, A.; Vurro, M. Stimulation of Orobanche ramosa seed germination by fusicoccin derivatives: A structure-activity relationship study. Phytochemistry 2006, 67, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Andolfi, A.; Cimmino, A.; Rubiales, D.; Evidente, A. Stimulation of seed germination of Orobanche species by ophiobolin a and fusicoccin derivatives. J. Agric. Food Chem. 2008, 56, 8343–8347. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aparicio, M.; Masi, M.; Maddau, L.; Cimmino, A.; Evidente, M.; Rubiales, D.; Evidente, A. Induction of haustorium development by sphaeropsidones in radicles of the parasitic weeds Striga and Orobanche. A structure-activity relationship study. J. Agric. Food Chem. 2016, 64, 5188–5196. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L. Parasitic plants. Ecology 1997, 78, 1612–1613. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Cristofaro, M.; Evidente, A. Cochliotoxin, a dihydropyranopyran-4,5-dione, and its analogues produced by Cochliobolus australiensis display phytotoxic activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Evidente, A. Chloromonilinic acids C and D, phytotoxic tetrasubstituted 3-chromanonacrylic acids isolated from Cochliobolus australiensis with potential herbicidal activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Górecki, M.; Mandoli, A.; Di Bari, L.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Clement, S.; Evidente, A. Pyriculins A and B, two monosubstituted hex-4-ene-2,3-diols and other phytotoxic metabolites produced by Pyricularia grisea isolated from buffelgrass (Cenchrus ciliaris). Chirality 2017, 29, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Andolfi, A.; Cimmino, A.; Evidente, A. Spirostaphylotrichin W, a spirocyclic γ-lactam isolated from liquid culture of Pyrenophora semeniperda, a potential mycoherbicide for cheatgrass (Bromus tectorum) biocontrol. Tetrahedron 2014, 70, 1497–1501. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Cimmino, A.; Andolfi, A.; Evidente, A. Pyrenophoric acid, a phytotoxic sesquiterpenoid penta-2,4-dienoic acid produced by a potential mycoherbicide Pyrenophora semeniperda. J. Nat. Prod. 2014, 77, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.E.; Masi, M.; Clement, S.; Davis, T.L.; Beckstead, J. Mycelial growth rate and toxin production in the seed pathogen Pyrenophora semeniperda: Resource trade-offs and temporally varying selection. Plant Pathol. 2015, 64, 1450–1460. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Application of Mosher’s method for absolute configuration assignment to bioactive plants and fungi metabolites. J. Pharm. Biomed. Anal. 2017, 144, 59–89. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2, 7, 8, 29–32, 43, 44, 150, 160, 167, 207, 216–224 are available from the authors. |



























© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Nocera, P.; Reveglia, P.; Cimmino, A.; Evidente, A. Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies. Molecules 2018, 23, 834. https://doi.org/10.3390/molecules23040834
Masi M, Nocera P, Reveglia P, Cimmino A, Evidente A. Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies. Molecules. 2018; 23(4):834. https://doi.org/10.3390/molecules23040834
Chicago/Turabian StyleMasi, Marco, Paola Nocera, Pierluigi Reveglia, Alessio Cimmino, and Antonio Evidente. 2018. "Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies" Molecules 23, no. 4: 834. https://doi.org/10.3390/molecules23040834




