Unprecedented Fluorescent Dinuclear CoII and ZnII Coordination Compounds with a Symmetric Bis(salamo)-Like Tetraoxime
Abstract
:1. Introduction
2. Results and Discussion
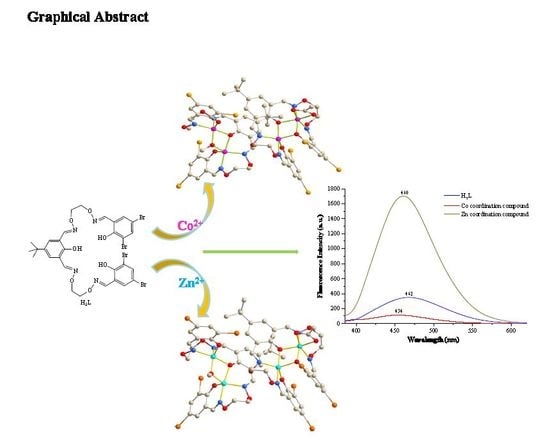
2.1. Crystal Structures of the Coordination Compounds 1 and 2
2.2. IR Spectra
2.3. Ultraviolet–Visible Spectroscopy (UV-Vis) Spectra
2.4. Fluorescence Spectra
3. Experimental
3.1. Materials and Physical Measurements
3.2. Preparation of Ligand H3L
3.3. Syntheses of the Coordination Compounds 1 and 2
3.4. X-ray Structure Determination of the Coordination Compounds 1 and 2
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.R.; Zhang, Y.H.; Wang, X.L. Synthesis, structure, antioxidation, and DNA-bindingstudies of a binuclear ytterbium(III) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. Res. Chem. Intermed. 2015, 41, 3375–3388. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, C.P.; Wang, F.; Peng, H.P.; Zhang, H.; Bai, Y.C. A new manganese(III) complex from bis(5-methylsalicylaldehyde)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidant activity and luminescence. J. Chin. Chem. Soc. 2015, 62, 1028–1034. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Pan, G.L.; Kong, J.; Shi, F.R.; Wang, X.L. Two lanthanide(III) complexes based on the schiff base N,N-Bis(salicylidene)-1,5-diamino-3-oxapentane: Synthesis, characterization, DNA-binding properties, and antioxidation. Z. Anorg. Allg. Chem. 2014, 640, 2062–2071. [Google Scholar] [CrossRef]
- Chai, L.Q.; Huang, J.J.; Zhang, H.S. An unexpected cobalt (III) complex containing a schiff base ligand: Synthesis, crystal structure, spectroscopic behavior, electrochemical property and SOD-like activity. Spectrochim. Acta Part A 2014, 131, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, L.C.; Ma, J.C.; Zhang, Y.; Zhang, J.; Dong, W.K. Syntheses, structures and spectral properties of mononuclear CuII and dimeric ZnII complexes based on an asymmetric Salamo-type N2O2 ligand. Z. Anorg. Allg. Chem. 2015, 641, 2520–2524. [Google Scholar] [CrossRef]
- Chai, L.Q.; Liu, G.; Zhang, Y.L.; Huang, J.J.; Tong, J.F. Synthesis, crystal structure, fluorescence, electrochemical property, and SOD-like activity of an unexpected nickel(II) complex with a quinazoline-type ligand. J. Coord. Chem. 2013, 66, 3926–3938. [Google Scholar] [CrossRef]
- Chai, L.Q.; Wang, G.; Sun, Y.X.; Dong, W.K.; Zhao, L.; Gao, X.H. Synthesis, crystal structure, and fluorescence of an unexpected dialkoxo-bridged dinuclear copper(II) complex with bis(Salen)-type tetraoxime. J. Coord. Chem. 2012, 65, 1621–1631. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, H.S.; Dong, W.K.; Zhao, Y.L. Synthesis of unsymmetrical ureas with coumarin and thiadiazole ring under microwave irradiation. Phosphorus Sulfur Silicon 2010, 185, 1332–1337. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Xiao, Z.R.; Li, X.; Liu, Y.A. Four polynuclear complexes based on a versatile salicylamide Salen-like ligand: Synthesis, structural variations and magnetic properties. Inorg. Chim. Acta 2015, 438, 232–244. [Google Scholar] [CrossRef]
- Liu, P.P.; Sheng, L.; Song, X.Q.; Xu, W.Y.; Liu, Y.A. Synthesis, structure and magnetic properties of a new one dimensional manganese coordination polymer constructed by a new asymmetrical ligand. Inorg. Chim. Acta 2015, 434, 252–257. [Google Scholar] [CrossRef]
- Song, X.Q.; Peng, Y.J.; Chen, G.Q.; Wang, X.R.; Liu, P.P.; Xu, W.Y. Substituted group-directed assembly of Zn(II) coordination complexes based on two new structural related pyrazolone based Salen ligands: Syntheses, structures and fluorescence properties. Inorg. Chim. Acta 2015, 427, 13–21. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Liu, Y.A.; Zhou, J.J.; Wang, X.L. Two dodecanuclear heterometallic [Zn6Ln6] clusters constructed by a multidentate salicylamide Salen-like ligand: Synthesis, structure, luminescence and magnetic properties. Dalton Trans. 2016, 45, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Bella, S.D.; Fragalà, I. Synthesis and second-order nonlinear optical properties of bis(salicylaldiminato)M(II) metalloorganic materials. Synth. Met. 2000, 115, 191. [Google Scholar] [CrossRef]
- Azam, M.; Al-Resayes, S.I.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Kumar, P. Seven-coordinated chiral uranyl(VI) Salen complex as effective catalyst for C–H bond activation of dialkylanilines under visible light. Polyhedron 2017, 124, 177–183. [Google Scholar] [CrossRef]
- Miyasaka, H.; Matsumoto, N.; Okawa, H.; Re, N.; Gallo, E. Complexes derived from the reaction of manganese(III) schiff base complexes and hexacyanoferrate(III): Syntheses, multidimensional network structures, and magnetic properties. J. Am. Chem. Soc. 1996, 118, 981–994. [Google Scholar] [CrossRef]
- Sharma, A.K.; Lloret, F.; Mukherjee, R. Phenolate- and acetate (both mu(2)-1,1 and mu(2)-1,3 modes)-bridged; Linear Co-3(II) and (Co2MnII)-Mn-II trimers: Magnetostructural studies. Inorg. Chem. 2013, 52, 4825–4833. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Li, Z.; Wu, M.C.; Chen, C.Y.; Zhang, J.W. Synthesis, crystal structure, antioxidation and DNA-binding properties of a dinuclear copper(II) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Coord. Chem. 2014, 67, 3054–3066. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.; Yuan, J.K.; Wang, H.; Pan, G.L.; Fan, X.Y.; Kong, J. A zinc(II) complex with tris(2-(N-methyl)benzimidazlylmethyl)amine and salicylate: Synthesis, crystal structure, and DNA-binding. J. Coord. Chem. 2012, 65, 2839–2851. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Wang, H.; Wang, X.L.; Bai, Y.C.; Zhang, Y.H. Study on synthesis, crystal structure, antioxidant and DNA-binding of mono-, di- and poly-nuclear lanthanides complexes with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Photochem. Photobiol. B 2014, 135, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.; Zhang, Y.H.; Wang, X.L. Preparation, structure, DNA-binding properties, and antioxidant activities of a homodinuclear erbium(III) complex with a pentadentate schiff base ligand. J. Chem. Res. 2014, 38, 211–217. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.W.; Zhang, Y.H.; Yang, Z.H.; Wu, H.L. Gadolinium(III) and dysprosium(III) complexes with a schiff base bis(N-salicylidene)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidation, and DNA-binding studies. J. Coord. Chem. 2015, 68, 1054–1071. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhang, S.T.; Ren, Z.L.; Dong, X.Y.; Wang, L. Synthesis, characterization, and crystal structure of a new supramolecular CdII complex with halogen-substituted Salen-type bisoxime. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 995–1000. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, L.; Zhao, T.H.; Liu, S.H.; Dong, X.T. Synthesis and crystal structure of a 3D supramolecular copper(II) complex with 1-(3-{[(E)-3-bromo-5-chloro-2-hydroxybenzylidene]amino}phenyl) ethanone oxime. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 509–513. [Google Scholar] [CrossRef]
- Chai, L.Q.; Tang, L.J.; Chen, L.C.; Huang, J.J. Structural, spectral, electrochemical and DFT studies of two mononuclear manganese(II) and zinc(II) complexes. Polyhedron 2017, 122, 228–240. [Google Scholar] [CrossRef]
- Chai, L.Q.; Huang, J.J.; Zhang, J.Y.; Li, Y.X. Two 1-D and 2-D cobalt(II) complexes: Synthesis, crystal structures, spectroscopic and electrochemical properties. J. Coord. Chem. 2015, 68, 1224–1237. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, K.Y.; Tang, L.J.; Zhang, J.Y.; Zhang, H.S. Two mono- and dinuclear Ni(II) complexes constructed from quinazoline-type ligands: Synthesis, X-ray structures, spectroscopic, electrochemical, thermal, and antimicrobial studies. Polyhedron 2017, 130, 100–107. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Dong, W.K.; Nabeshima, T. Oxime-based Salen-type tetradentate ligands with high stability against imine metathesis reaction. J. Org. Chem. 2005, 70, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Liu, L.Z.; Dong, W.K.; Zhang, J.; Zhang, Y. Three multinuclear Co(II), Zn(II) and Cd(II) complexes based on a single-armed Salamo-type bisoxime: Syntheses, structural characterizations and fluorescent properties. J. Coord. Chem. 2017, 70, 1936–1952. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Self-assembled zinc(II)-lanthanide(III) heteromultinuclear complexes constructed from 3-MeO Salamo ligand: Syntheses, structures and luminescent properties. Cryst. Growth Des. 2016, 16, 6903–6914. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.T.; Dong, Y.J.; Zhang, Y.; Wang, Z.K. Construction of mononuclear copper(II) and trinuclear cobalt(II) complexes based on asymmetric Salamo-type ligands. Z. Anorg. Allg. Chem. 2016, 642, 189–196. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, X.; Yang, C.J.; Zhao, M.M.; Li, G.; Dong, X.Y. Syntheses and crystal structures of 5-methoxy-6′-hydroxy-2,2′-[ethylenedioxybis(nitrilomethylidyne)]diphenol and its tetranuclear zinc(II) complex. Chin. J. Inorg. Chem. 2014, 30, 710–716. [Google Scholar]
- Dong, W.K.; Lan, P.F.; Zhou, W.M.; Zhang, Y. An unexpected dinuclear Cu(II) complex with a bis(Salamo) chelating ligand: Synthesis, crystal structure, and photophysical properties. J. Coord. Chem. 2016, 69, 149–159. [Google Scholar]
- Sun, Y.X.; Gao, X.H. Synthesis, characterization, and crystal structure of a new CuII complex with Salen-type ligand. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2011, 41, 973–978. [Google Scholar] [CrossRef]
- Sun, Y.X.; Wang, L.; Dong, X.Y.; Ren, Z.L.; Meng, W.S. Synthesis, characterization, and crystal structure of a supramolecular CoII complex containing Salen-type bisoxime. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 599–603. [Google Scholar] [CrossRef]
- Liu, Y.A.; Wang, C.Y.; Zhang, M.; Song, X.Q. Structures and magnetic properties of cyclic heterometallic tetranuclear clusters. Polyhedron 2017, 127, 278–286. [Google Scholar] [CrossRef]
- Liu, P.P.; Wang, C.Y.; Zhang, M.; Song, X.Q. Pentanuclear sandwich-type ZnII-LnIII clusters based on a new Salen-like salicylamide ligand: Structure, near-infrared emission and magnetic properties. Polyhedron 2017, 129, 133–140. [Google Scholar] [CrossRef]
- Zhao, L.; Dang, X.T.; Chen, Q.; Zhao, J.X.; Wang, L. Synthesis, crystal structure and spectral properties of a 2D supramolecular copper(II) complex with 1-(4-{[(E)-3-ethoxyl-2-hydroxybenzylidene]amino}phenyl)ethanone oxime. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2013, 43, 1241–1246. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L. An infinite 2D supramolecular cobalt(II) complex based on an asymmetric Salamo-type ligand: Synthesis, crystal structure, and spectral properties. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2016, 46, 1095–1101. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L. Synthesis, structure and spectroscopic properties of the trinuclear cobalt(II) and nickel(II) complexes based on 2-hydroxynaphthaldehyde and bis(aminooxy)alkane. Spectrochim. Acta Part A 2015, 135, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Sun, Y.X.; Dong, W.K.; Tang, X.L.; Gao, X.H. A supramolecular copper(II) complex bearing Salen-type bisoxime ligand: Synthesis, structural characterization, and thermal property. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2012, 42, 1303–1308. [Google Scholar] [CrossRef]
- Akine, S.; Utsuno, F.; Taniguchi, T.; Nabeshima, T. Dinuclear complexes of the N2O2 oxime chelate ligand with zinc(II)–lanthanide(III) as a selective sensitization system for Sm3+. Eur. J. Inorg. Chem. 2010, 49, 3143–3152. [Google Scholar] [CrossRef]
- Wang, L.; Hao, J.; Zhai, L.X.; Zhang, Y.; Dong, W.K. Synthesis, crystal structure, luminescence, electrochemical and antimicrobial properties of bis(Salamo)-based Co(II) complex. Crystals 2017, 7, 277. [Google Scholar] [CrossRef]
- Wang, B.J.; Dong, W.K.; Zhang, Y.; Akogun, S.F. A novel relay-sensor for highly sensitive and selective detection of Zn2+/Pic− and fluorescence on/off switch response of H+/OH−. Sens. Actuators B 2017, 247, 254–264. [Google Scholar] [CrossRef]
- Wang, F.; Gao, L.; Zhao, Q.; Zhang, Y.; Dong, W.K.; Ding, Y.J. A highly selective fluorescent chemosensor for CN− based on a novel bis(Salamo)-type tetraoxime ligand. Spectrochim. Acta A 2018, 190, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Akogun, S.F.; Zhou, W.M.; Dong, W.K. Tetranuclear Zn(II) complex based on an asymmetrical Salamo-type chelating ligand: Synthesis, structural characterization, and fluorescence property. J. Chin. Chem. Soc. 2017, 64, 412–419. [Google Scholar] [CrossRef]
- Dong, Y.J.; Li, X.L.; Zhang, Y.; Dong, W.K. A highly selective visual and fluorescent sensor for Pb2+ and Zn2+ and crystal structure of Cu2+ complex based-on a novel single-armed Salamo-type bisoxime. Supramol. Chem. 2017, 29, 518–527. [Google Scholar] [CrossRef]
- Li, L.L.; Dong, W.K.; Zhang, Y.; Akogun, S.F.; Xu, L. Syntheses, structures and catecholase activities of homo- and hetero-trinuclear cobalt(II) complexes constructed from an acyclic naphthalenediol-based bis(Salamo)-type ligand. Appl. Organomet. Chem. 2017, 31, e3818. [Google Scholar] [CrossRef]
- Dong, Y.J.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhang, L.S. Three asymmetric Salamo-type copper(II) and cobalt(II) complexes: Syntheses, structures, fluorescent properties. Polyhedron 2017, 123, 305–315. [Google Scholar] [CrossRef]
- Dong, Y.J.; Ma, J.C.; Zhu, L.C.; Dong, W.K.; Zhang, Y. Four 3d–4f heteromultinuclear zinc(II)–lanthanide(III) complexes constructed from a distinct hexadentate N2O2-type ligand: Syntheses, structures and photophysical properties. J. Coord. Chem. 2017, 70, 103–115. [Google Scholar] [CrossRef]
- Dong, W.K.; Akogun, S.F.; Zhang, Y.; Dong, X.Y. A reversible “turn-on” fluorescent sensor for selective detection of Zn2+. Sens. Actuators B 2017, 238, 723–734. [Google Scholar] [CrossRef]
- Peng, Y.D.; Li, X.Y.; Kang, Q.P.; An, G.X.; Zhang, Y.; Dong, W.K. Synthesis and fluorescence properties of asymmetrical Salamo-type tetranuclear zinc(II) complex. Crystals 2018, 8, 107. [Google Scholar] [CrossRef]
- Li, X.Y.; Kang, Q.P.; Liu, L.Z.; Ma, J.C.; Dong, W.K. Trinuclear Co(II) and mononuclear Ni(II) Salamo-type bisoxime coordination compounds. Crystals 2018, 8, 43. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, X.L.; Wang, L.; Zhang, Y.; Ding, Y.J. A new application of Salamo-type bisoximes: As a relay-sensor for Zn2+/Cu2+ and its novel complexes for successive sensing of H+/OH−. Sens. Actuators B 2016, 229, 370–378. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.; Zhang, Y.; Li, N. Novel multinuclear transition metal(II) complexes based on an asymmetric Salamo-type ligand: Syntheses, structure characterizations and fluorescent properties. Inorg. Chim. Acta 2016, 444, 95–102. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, G.; Wang, Z.K.; Dong, X.Y. A novel trinuclear cobalt(II) complex derived from an asymmetric Salamo-type N2O3 bisoxime chelate ligand: Synthesis, structure and optical properties. Spectrochim. Acta Part A 2014, 133, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, W.K.; Zhang, H.; Zhang, Y.; Sun, Y.X. Structural variation and luminescence properties of triand dinuclear CuII and ZnII complexes constructed from a naphthalenediol-based bis(Salamo)-type ligand. Cryst. Growth Des. 2017, 17, 3636–3648. [Google Scholar] [CrossRef]
- Akine, S. Novel ion recognition systems based on cyclic and acyclic oligo(Salen)-type ligands. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 25–54. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.C.; Dong, W.K.; Zhu, L.C.; Zhang, Y. A novel self–assembled nickel(II)–cerium(III) heterotetranuclear dimer constructed from N2O2-type bisoxime and terephthalic acid: Synthesis, structure and photophysical properties. Z. Anorg. Allg. Chem. 2016, 642, 834–839. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, W.K.; Zhang, Y.; Akogun, S.F. Naphthalenediol-based bis(Salamo)-type homo- and heterotrinuclear cobalt(II) complexes: Syntheses, structures and magnetic properties. Polyhedron 2017, 133, 279–293. [Google Scholar] [CrossRef]
- Dong, X.Y.; Gao, L.; Wang, F.; Zhang, Y.; Dong, W.K. Tri- and mono-nuclear zinc(II) complexes based on half- and mono-Salamo chelating ligands. Crystals 2017, 7, 267. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, L.; Gao, L.; Zhang, Y.; Akogun, S.F.; Dong, W.K. Syntheses, crystal structures and catalytic activities of two solvent-induced homotrinuclear Co(II) complexes with a naphthalenediol-based bis(Salamo)-type tetraoxime ligand. RSC Adv. 2017, 7, 35905–35916. [Google Scholar] [CrossRef]
- Dong, X.Y.; Li, X.Y.; Liu, L.Z.; Zhang, H.; Ding, Y.J.; Dong, W.K. Tri- and hexanuclear heterometallic Ni(II)–M(II) (M = Ca, Sr and Ba) bis(Salamo)-type complexes: Synthesis, structure and fluorescence properties. RSC Adv. 2017, 7, 48394–48403. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; The crystal and molecular structure of aqua[l,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The rich stereochemistry of eight-vertex polyhedra: A continuous shape measures study. Chemistry 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.S.; Dong, W.K.; Zhang, Y.; Chen, L.; Ding, Y.J. Four Salamo-type 3d–4f hetero-bimetallic [ZnIILnIII] complexes: Syntheses, crystal structures, and luminescent and magnetic properties. New J. Chem. 2017, 41, 4966–4973. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.Y.; Zhao, Q.; Li, L.H.; Dong, W.K. Fluorescence properties of heterotrinuclear Zn(II)-M(II) (M = Ca, Sr and Ba) bis(Salamo)-type complexes. RSC Adv. 2017, 7, 48730–48737. [Google Scholar] [CrossRef]
- Hao, J.; Li, L.H.; Zhang, J.T.; Akogun, S.F.; Wang, L.; Dong, W.K. Four homo- and hetero-bismetallic 3d/3d-2s complexes constructed from a naphthalenediol-based acyclic bis(Salamo)-type tetraoxime ligand. Polyhedron 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Percy, G.; Thornton, D. Infrared spectra of N-aryl salicylaldimine complexes substituted in both aryl rings. J. Inorg. Nucl. Chem. 1973, 35, 2319–2327. [Google Scholar] [CrossRef]
- Gao, L.; Liu, C.; Wang, F.; Dong, W.K. Tetra-, penta- and hexa-coordinated transition metal complexes constructed from coumarin-containing N2O2 ligand. Crystals 2018, 8, 77. [Google Scholar] [CrossRef]
- Song, X.Q.; Cheng, G.Q.; Wang, X.R.; Xu, W.Y.; Liu, P.P. Structure-based description of a step-by-step synthesis of heterodinuclear ZnIILnIII complexes and their luminescence properties. Inorg. Chim. Acta 2015, 425, 145–153. [Google Scholar] [CrossRef]
- Dong, W.K.; Du, W.; Zhang, X.Y.; Li, G.; Dong, X.Y. Synthesis, crystal structure and spectral properties of a supramolecular trinuclear nickel(II) complex with 5-methoxy-4′-bromo-2,2′-[ethylenedioxybis(nitrilomethylidyne)]diphenol. Spectrochim. Acta Part A 2014, 132, 588–593. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |









| Coordination Compound 1 | |||||
|---|---|---|---|---|---|
| Bonds Lengths (Å) | Bonds Lengths (Å) | Bonds Lengths (Å) | |||
| Co1-O1 | 1.952(3) | Co2-N3 | 2.060(3) | Co3-O16 | 2.138(3) |
| Co1-O8 | 1.961(3) | Co2-O4 | 2.069(3) | Co3-O17 | 2.198(3) |
| Co1-N2 | 2.033(3) | Co2-N4 | 2.083(4) | Co4-O15 | 1.917(3) |
| Co1-N1 | 2.045(3) | Co3-O12 | 2.021(3) | Co4-O9 | 2.022(3) |
| Co1-O4 | 2.071(3) | Co3-N5 | 2.047(4) | Co4-N7 | 2.027(3) |
| Co2-O7 | 1.954(3) | Co3-N6 | 2.063(3) | Co4-O12 | 2.086(3) |
| Co2-O8 | 1.961(3) | Co3-O9 | 2.074(3) | Co4-N8 | 2.139(3) |
| Coordination Compound 2 | |||||
| Bonds Lengths (Å) | Bonds Lengths (Å) | Bonds Lengths (Å) | |||
| Zn1-O17 | 1.954(5) | Zn2-O16 | 1.951(5) | Zn3-N6 | 2.115(5) |
| Zn1-O14 | 2.082(4) | Zn2-N3 | 2.103(6) | Zn3-N5 | 2.103(5) |
| Zn1-O15 | 1.978(4) | Zn2-N4 | 2.111(5) | Zn4-O4 | 2.096(4) |
| Zn1-N1 | 2.088(6) | Zn3-O4 | 2.047(4) | Zn4-O9 | 1.941(4) |
| Zn1-N2 | 2.101(5) | Zn3-O3 | 2.056(4) | Zn4-O3 | 2.027(4) |
| Zn2-O14 | 2.057(4) | Zn3-O7 | 2.205(5) | Zn4-N8 | 2.031(5) |
| Zn2-O15 | 1.991(5) | Zn3-O8 | 2.138(5) | Zn4-N7 | 2.180(5) |
| Coordination Compound 1 | |||||
|---|---|---|---|---|---|
| D‒X···A | d(D‒X) | d(X···A) | d(D···A) | ∠D‒X···A | Symmetry Code |
| C9‒H9A···O13 | 0.97 | 2.44 | 3.231(6) | 138 | |
| C10‒H10···O15 | 0.93 | 2.43 | 3.130(5) | 132 | |
| C40‒H40A···Br8 | 0.97 | 2.90 | 3.776(6) | 151 | −x,1−y,1−z |
| C43‒H43···O7 | 0.93 | 2.60 | 3.477(6) | 158 | |
| C49‒H49A···O16 | 0.97 | 2.46 | 3.379(6) | 159 | |
| C8‒H8A···Br8 | 0.97 | 3.92 | 3.019(5) | 154 | |
| C61‒H61A···Br2 | 0.96 | 2.99 | 3.280(6) | 99 | |
| C36‒H36···Br7 | 0.93 | 3.81 | 3.044(5) | 140 | |
| C39‒H39B···Br6 | 0.97 | 2.96 | 3.895(5) | 161 | |
| Coordination Compound 2 | |||||
| D‒X···A | d(D‒X) | d(X···A) | d(D···A) | ∠D‒X···A | Symmetry Code |
| C6‒H6···O16 | 0.93 | 2.52 | 3.394(8) | 157 | |
| C11‒H11···O16 | 0.93 | 2.57 | 3.439(7) | 156 | |
| C22‒H22A···O8 | 0.97 | 2.43 | 3.374(8) | 163 | |
| C44‒H44···O9 | 0.93 | 2.40 | 3.070(9) | 129 | |
| C55‒H55···O10 | 0.93 | 2.59 | 3.297(9) | 133 | [1−x,1−y,1−z] |
| Coordination Compound | ν(C=N) | ν(Ar–O) | ν(Co–O) | ν(Co–N) |
|---|---|---|---|---|
| H3L | 1611 | 1265 | ||
| 1 | 1619 | 1258 | 447 | 512 |
| 2 | 1621 | 1261 | 453 | 519 |
| Coordination Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C63H60Br8Co4N8O17 | C63H60Br8Zn4N8O17 |
| Molecular weight | 2076.19 | 2101.95 |
| Color | Brown | Bright-yellow |
| Crystal size, mm | 0.22 × 0.18 × 0.16 | 0.22 × 0.18 × 0.17 |
| Habit | Block-shaped | Block-shaped |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| Unit cell dimension | ||
| a, Å | 15.244(2) | 13.4501(6) |
| b, Å | 18.674(3) | 18.6963(9) |
| c, Å | 21.356(3) | 19.6467(8) |
| α, ° | 109.512(4) | 72.7450(10) |
| β, ° | 97.335(4) | 72.5280(10) |
| γ, ° | 109.429(4) | 88.801(2) |
| Volume, Å3 | 5205.4(14) | 4487.9(3) |
| Z | 2 | 2 |
| Calculated density, mg·m−3 | 1.325 | 1.555 |
| Absorption coefficient, mm−1 | 3.747 | 4.675 |
| F (000) | 2036 | 2060 |
| θ range for data collection, ° | 1.050 to 27.000 | 2.224 to 25.010 |
| h/k/l (min, max) | −19, 16/−22, 23/−27, 26 | −11, 15/−22, 21/−23, 23 |
| Reflections collected | 37,783 | 32,780 |
| Completeness | 96.6% | 99.4% |
| Data/restraints/parameters | 22,025/1/909 | 15,711/0/909 |
| Final R indices [I > 2σ(I)] | R1 = 0.0438, wR2 = 0.0932 | R1 = 0.0523, wR2 = 0.1353 |
| R indices (all data) | R1 = 0.0762, wR2 = 0.0977 | R1 = 0.0888, wR2 = 0.1557 |
| Largest diff. peak and hole (e·Å−3) | 1.677, −0.861 | 1.434, −0.914 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-W.; Liu, L.-Z.; Wang, F.; Dong, W.-K. Unprecedented Fluorescent Dinuclear CoII and ZnII Coordination Compounds with a Symmetric Bis(salamo)-Like Tetraoxime. Molecules 2018, 23, 1141. https://doi.org/10.3390/molecules23051141
Zhang L-W, Liu L-Z, Wang F, Dong W-K. Unprecedented Fluorescent Dinuclear CoII and ZnII Coordination Compounds with a Symmetric Bis(salamo)-Like Tetraoxime. Molecules. 2018; 23(5):1141. https://doi.org/10.3390/molecules23051141
Chicago/Turabian StyleZhang, Lin-Wei, Ling-Zhi Liu, Fei Wang, and Wen-Kui Dong. 2018. "Unprecedented Fluorescent Dinuclear CoII and ZnII Coordination Compounds with a Symmetric Bis(salamo)-Like Tetraoxime" Molecules 23, no. 5: 1141. https://doi.org/10.3390/molecules23051141