Glycoside Mimics from Glycosylamines: Recent Progress
Abstract
:1. Introduction
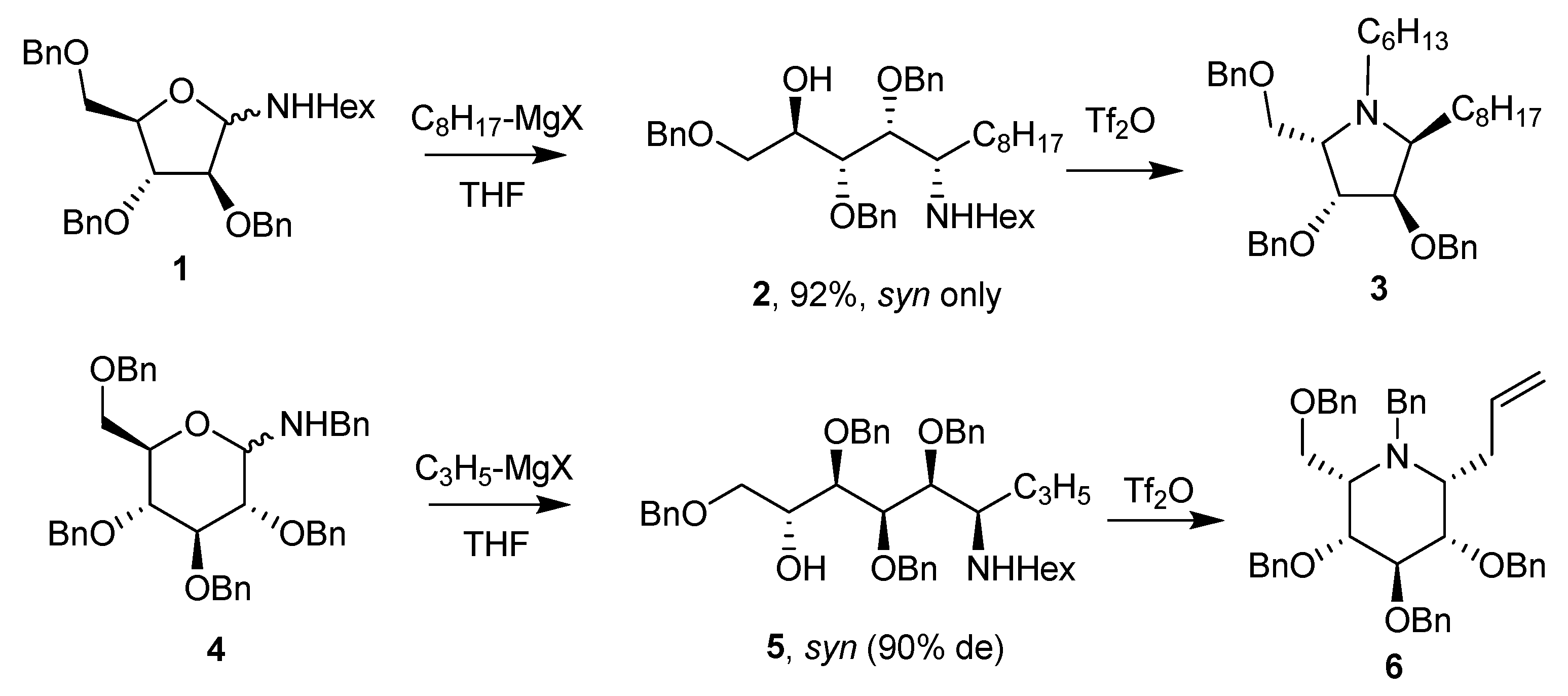
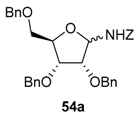
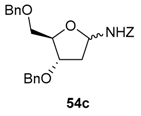
2. N-(Benzyl)- and Other N-(Alkyl)-N-Glycosides
3. N-Benzyl-N-Glycosylhydroxylamines
3.1. Synthesis
3.2. Addition Reactions
3.3. Cyclizations
4. N-(Alkoxycarbonyl)-N-Glycosides
4.1. Synthesis
4.2. Addition Reactions
4.3. Cyclization Reactions
4.4. Miscellaneous
5. N-(tert-Butanesulfinyl) Glycosylamines
5.1. Synthesis
5.2. Addition Reactions
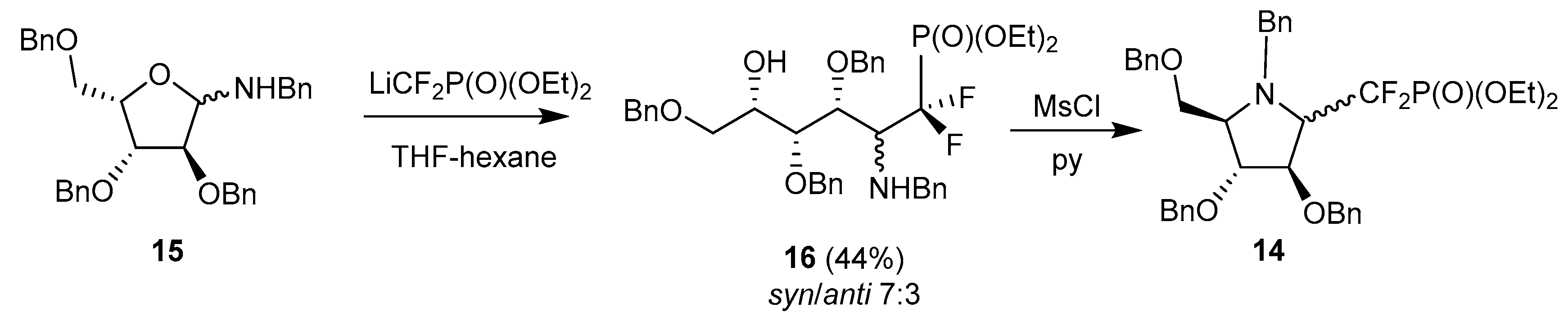
5.3. Addition of Grignard Reagents and Cyclizations
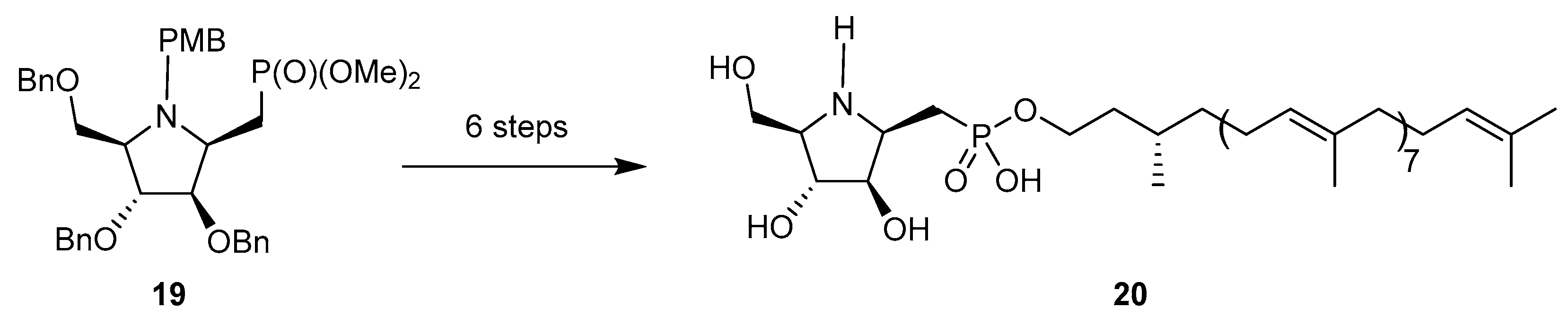
5.4. Addition of M-CF2P(O)(OEt)2 Metalated Species and Cyclizations
6. Conclusions
Funding
Conflicts of Interest
References
- Carbohydrates in Chemistry and Biology; Ernst, B.; Hart, G.W.; Sinaÿ, P. (Eds.) Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Bertozzi, C.R.; Kiessling, L.L. Chemical Glycobiology. Science 2001, 291, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Hudak, J.E.; Bertozzi, C.R. Glycotherapy: New Advances Inspire a Reemergence of Glycans in Medicine. Chem. Biol. 2014, 21, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Davis, B.G. Realizing the Promise of Chemical Glycobiology. Chem. Sci. 2013, 4, 3381–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Sugar Code: Fundamentals of Glycosciences; Gabius, H.-J. (Ed.) Wiley-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-32089-9. [Google Scholar]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 2017; ISBN 9781621821328. [Google Scholar]
- Sattin, S.; Bernardi, A. Design and synthesis of glycomimetics. Carbohydr. Chem. 2016, 41, 1–25. [Google Scholar] [CrossRef]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Biochemistry of Glycoconjugate Glycans. In Comprehensive Glycosciences: From Chemistry to Systems Biology; Kamerling, J.P.; Boons, G.J.; Lee, Y.C.; Suzuki, A.; Taniguchi, N.; Voragen, A.G.J. (Eds.) Elsevier: Oxford, UK, 2007; Volume 3, ISBN 9780444519672. [Google Scholar]
- Spiro, R.G. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R. [Google Scholar] [CrossRef] [PubMed]
- Gamblin, D.P.; Scanlan, E.M.; Davis, B.G. Glycoprotein Synthesis: An Update. Chem. Rev. 2009, 109, 131–163. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Danishefsky, S.J. Recent Departures in the Synthesis of Peptides and Glycopeptides. Tetrahedron 2009, 65, 9047–9065. [Google Scholar] [CrossRef] [PubMed]
- Larkin, A.; Imperiali, B. The Expanding Horizons of Asparagine-Linked Glycosylation. Biochemistry 2011, 50, 4411–4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef] [PubMed]
- Takeda, U.; Okada, T.; Takagi, M.; Gomi, S.; Itoh, J.; Sezaki, M.; Ito, M.; Miyadoh, S.; Shomura, T. SF2446, New Benzo[a]naphthacene Quinone Antibiotics. I. Taxonomy and Fermentation of the Producing Strain, Isolation and Characterization of Antibiotics. J. Antibiot. 1988, 41, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Gomi, S.; Sazaki, M.; Itoh, J.; Sezaki, M. SF2446, New Benzo[a]naphthacene Quinone Antibiotics. II. The Structural Elucidation. J. Antibiot. 1988, 41, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Maskey, R.P.; Grün-Wollny, I.; Fiebig, H.H.; Laatsch, H. Akashins A, B, and C: Novel Chlorinated Indigoglycosides from Streptomyces sp. GW 48/1497. Angew. Chem. Int. Ed. 2002, 41, 597–599. [Google Scholar] [CrossRef]
- Link, J.T.; Raghavan, S.; Gallant, M.; Danishefsky, S.J.; Chou, T.C.; Ballas, L.M. Staurosporine and ent-Staurosporine: The First Total Syntheses, Prospects for a Regioselective Approach, and Activity Profiles. J. Am. Chem. Soc. 1996, 118, 2825–2842. [Google Scholar] [CrossRef]
- Belmokhtar, C.A.; Hillion, J.; Ségal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonsson, A.; Persson, J.L. Induction of Apoptosis by Staurosporine Involves the Inhibition of Expression of the Major Cell Cycle Proteins at the G2/M Checkpoint Accompanied by Alteration in Erk and Akt Kinase Activities. Anticancer Res. 2009, 29, 2893–2898. [Google Scholar] [PubMed]
- Bush, J.A.; Long, B.H.; Catino, J.J.; Bradner, W.T.; Tomita, K. Production and Biological Activity of Rebeccamycin, a Novel Antitumor Agent. J. Antibiot. 1987, 40, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Snipes, C.E.; Duebelbeis, D.O.; Olson, M.; Hahn, D.R.; Dent, W.H., III; Gilbert, J.R.; Werk, T.L.; Davis, G.E.; Lee-Lu, R.; Graupner, P.R. The Ansacarbamitocins: Polar Ansamitocin Derivatives. J. Nat. Prod. 2007, 70, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Eklund, E.A.; Bode, L.; Freeze, H.H. Diseases associated with carbohydrates/glycoconjugates. In Comprehensive Glycoscience. From Chemistry to Systems Biology, 1st ed.; Kamerling, J.P., Boons, G.J., Lee, Y.C., Suzuki, A., Taniguchi, N., Voragen, A.G.J., Eds.; Elsevier: Oxford, UK, 2007; Volume 4, pp. 339–371. ISBN 0-444-52746-X. [Google Scholar]
- Legler, G. Glycoside Hydrolases: Mechanistic Information from Studies with Reversible and Irreversible Inhibitors. Adv. Carbohydr. Chem. Biochem. 1990, 48, 319–384. [Google Scholar] [CrossRef] [PubMed]
- Isbell, H.S.; Frush, H.L. Mutarotation, Hydrolysis, and Rearrangement Reactions of Glycosylamines. J. Org. Chem. 1958, 23, 1309–1319. [Google Scholar] [CrossRef]
- Compain, P.; Chagnault, V.; Martin, O.R. Tactics and strategies for the synthesis of iminosugar C-glycosides: A review. Tetrahedron Asymmetry 2009, 20, 672–711. [Google Scholar] [CrossRef]
- Iminosugars: From Synthesis to Therapeutic Applications; Compain, P.; Martin, O.R. (Eds.) John Wiley & Sons: Chichester, UK, 2007; ISBN 9780470033913. [Google Scholar]
- Horne, G.; Wilson, F.X.; Tinsley, J.; Williams, D.H.; Storer, R. Iminosugars past, present and future: Medicines for tomorrow. Drug Discov. Today 2011, 16, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.J.; Kato, A.; Yu, C.-Y.; Fleet, G.W. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Asano, N. Iminosugars: The Potential of Carbohydrate Analogs. In Carbohydrate Chemistry: State of the Art and Challenges for Drug Development; Cipolla, L., Ed.; Imperial College Press: London, UK, 2016; pp. 279–301. ISBN 978-1-78326-720-0. [Google Scholar]
- Germain, D.; Giugliani, R.; Hughes, D.; Mehta, A.; Nicholls, K.; Barisoni, L.; Jennette, C.J.; Bragat, A.; Castelli, J.; Sitaraman, S.; et al. Safety and Pharmacodynamic effects of a pharmacological chaperone on α-galactosidase A activity and globotriaosylceramide clearance in Fabry disease: Report from two phase 2 clinical studies. Orphanet J. Rare Dis. 2012, 7, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Spencer, C.M. Miglitol: A review of its therapeutic potential in type 2 diabetes mellitus. Drugs 2000, 59, 521–549. [Google Scholar] [CrossRef] [PubMed]
- Canda, E.; Kose, M.; Kagnici, M.; Ucar, S.K.; Sozmen, E.Y.; Coker, M. Patients with Gaucher type 1: Switching from imiglucerase to miglustat therapy. Blood Cells Mol. Dis. 2018, 68, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Boto, A. Nucleoside Analogues: Synthesis and Biological Properties of Azanucleoside Derivatives. Eur. J. Org. Chem. 2014, 2201–2220, and references cited therein. [Google Scholar] [CrossRef]
- Singh, V.; Evans, G.B.; Lenz, D.H.; Mason, J.M.; Clinch, K.; Mee, S.; Painter, G.F.; Tyler, P.C.; Furneaux, R.H.; Lee, J.E.; et al. Femtomolar Transition State Analogue Inhibitors of 5′-Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase from Escherichia coli. J. Biol. Chem. 2005, 280, 18265–18273. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Singh, V.; Evans, G.B.; Tyler, P.C.; Furneaux, R.H.; Cornell, K.A.; Riscoe, M.K.; Schramm, V.L.; Howell, P.L. Structural Rationale for the Affinity of Pico- and Femtomolar Transition State Analogues of Escherichia coli 5′-Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase. J. Biol. Chem. 2005, 280, 18274–18282. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, E.M.; Garcia Fernández, J.M.; Ortiz Mellet, C. Glycomimetic-based pharmacological chaperones for lysosomal storage disorders: Lessons from Gaucher, GM1-gangliosidosis and Fabry diseases. Chem. Commun. 2016, 52, 5497–5515. [Google Scholar] [CrossRef] [PubMed]
- Bergeron-Brlek, M.; Goodwin-Tindall, J.; Cekic, N.; Roth, C.; Zandberg, W.F.; Shan, X.; Varghese, V.; Chan, S.; Davies, G.J.; Vocadlo, D.J.; et al. A Convenient Approach to Stereoisomeric Iminocyclitols: Generation of Potent Brain-Permeable OGA Inhibitors. Angew. Chem. Int. Ed. 2015, 54, 15429–15433. [Google Scholar] [CrossRef] [PubMed]
- Bergeron-Brlek, M.; Meanwell, M.; Britton, R. Direct synthesis of imino-C-nucleoside analogues and other biologically active iminosugars. Nat. Commun. 2015, 6, 6903–6908. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, M.; Sutherland, M.; Britton, R. Application of sequential proline-catalyzed α-chlorination and aldol reactions in the total synthesis of 1-deoxygalactonojirimycin. Can. J. Chem. 2018, 96, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Behr, J.-B.; Plantier-Royon, R. Addition of organometallics to aldimines, aldoximes, and aldononitriles: A key step towards the synthesis of azasugars. Recent Res. Dev. Org. Chem. 2006, 10, 1–30. [Google Scholar] [CrossRef]
- Carcano, M.; Nicotra, F.; Panza, L.; Russo, G. A New Procedure for the Synthesis of d-Glucosamine α-C-Glycosides. J. Chem. Soc. Chem. Commun. 1989, 287–289. [Google Scholar] [CrossRef]
- Lay, L.; Nicotra, F.; Paganini, A.; Pangrazio, C.; Panza, L. A New Procedure for the Synthesis of Azasugars. Tetrahedron Lett. 1993, 34, 4555–4558. [Google Scholar] [CrossRef]
- Cipolla, L.; Lay, L.; Nicotra, F.; Pangrazio, C.; Panza, L. Synthesis of Azasugars by Grignard Reactions on Glycosylamines. Tetrahedron 1995, 51, 4679–4690. [Google Scholar] [CrossRef]
- Cipolla, L.; La Ferla, B.; Peri, F.; Nicotra, F. A new procedure for the synthesis of C-glycosides of nojirimycin. Chem. Commun. 2000, 1289–1290. [Google Scholar] [CrossRef]
- Cipolla, L.; Palma, A.; La Ferla, B.; Nicotra, F. Synthesis of nojirimycin C-glycosides. J. Chem. Soc. Perkin Trans. 1 2002, 2161–2165. [Google Scholar] [CrossRef]
- Von der Osten, C.H.; Sinskey, A.J.; Barbas, C.F., III; Pederson, R.L.; Wang, Y.-F.; Wong, C.-H. Use of a Recombinant Bacterial Fructose-1,6-diphosphate Aldolase in Aldol Reactions: Preparative Syntheses of 1-Deoxynojirimycin, 1-Deoxymannojirimycin, 1,4-Dideoxy-1,4-imino-D-arabinitol, and Fagomine. J. Am. Chem. Soc. 1989, 111, 3924–3927. [Google Scholar] [CrossRef]
- Wennekes, T.; Overkleeft, H.S. Synthesis and Evaluation of Lipophilic Aza-C-glycosides as Inhibitors of Glucosylceramide Metabolism. Eur. J. Org. Chem. 2010, 1258–1283. [Google Scholar] [CrossRef]
- Lahiri, R.; Suman Reddy, Y.; Kulkarni, S.A.; Vankar, Y.D. Synthesis of unnatural indolizidines, pyrrolizidine and C-alkyl iminosugars from sugar derived hemiaminals. RSC Adv. 2013, 3, 23242–23254. [Google Scholar] [CrossRef]
- Wang, H.Y.; Kato, A.; Kinami, K.; Li, Y.-X.; Fleet, G.W.J.; Yu, C.-Y. Concise synthesis of calystegines B2 and B3 via intramolecular Nozaki–Hiyama–Kishi reaction. Org. Biomol. Chem. 2016, 14, 4885–4896. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Peczuh, M.W. Access to Ring-Expanded Analogues of 2-Amino Sugars. Org. Lett. 2009, 11, 4482–4484. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Peczuh, M.W. Expanding the Scope of Aminosugars: Synthesis of 2-Amino Septanosyl Glycoconjugates Using Septanosyl Fluoride Donors. Chemistry 2011, 17, 7357–7365. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.B.; Chagnault, V.; Postel, D. Synthesis of glycosylamines and glyconamides using molecular iodine. Tetrahedron 2013, 69, 542–550. [Google Scholar] [CrossRef]
- Prasada Rao, J.; Venkataswara Rao, B.; Lakshmi Swarnalatha, J. A new stereoselective approach for N-benzyl amino(hydroxymethyl)-cyclopentitols using RCM. Tetrahedron Lett. 2010, 51, 3083–3087. [Google Scholar] [CrossRef]
- Srinivas Rao, G.; Venkataswara Rao, B. A common and stereoselective strategy for the synthesis of N,O,O,O-tetra-acetyl d-ribo-(2S,3S,4R)-phytosphingosine and 2-epi-jaspine B. Tetrahedron Lett. 2011, 52, 4861–4864. [Google Scholar] [CrossRef]
- Rajender, A.; Prasada Rao, J.; Venkataswara Rao, B. A Divergent and Stereoselective Approach for the Syntheses of Some Polyhydroxylated Indolizidine and Pyrrolizidine Iminosugars. Eur. J. Org. Chem. 2013, 1749–1757. [Google Scholar] [CrossRef]
- Chirke, S.S.; Rajender, A.; Lakshmi, J.K.; Venkataswara Rao, B. A divergent, short and stereoselective approach to pyrrolidine iminosugars: Synthesis of 1,4-dideoxy-1,4-imino-derivatives of d-allitol, d-ribitol, ethyl-erythritol and (–)-2,3-trans-3-4-cis-dihydroxyproline. Tetrahedron Lett. 2015, 56, 1218–1221. [Google Scholar] [CrossRef]
- Kotland, A.; Accadbled, F.; Robeyns, K.; Behr, J.B. Synthesis and Fucosidase Inhibitory Study of Unnatural Pyrrolidine Alkaloid 4-epi-(+)-Codonopsinine. J. Org. Chem. 2011, 76, 4094–4098. [Google Scholar] [CrossRef] [PubMed]
- Hottin, A.; Dubar, F.; Steenackers, A.; Delannoy, P.; Biot, C.; Behr, J.-B. Iminosugar–ferrocene conjugates as potential anticancer agents. Org. Biomol. Chem. 2012, 10, 5592–5597. [Google Scholar] [CrossRef] [PubMed]
- Hottin, A.; Wright, D.W.; Davies, G.J.; Behr, J.-B. Exploiting the Hydrophobic Terrain in Fucosidases with Aryl-Substituted Pyrrolidine Iminosugars. ChemBioChem 2015, 16, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.-J.; Ye, J.-L.; Zhang, H.-K.; Huang, P.-Q. An unexpected high erythro-selection in the Grignard reaction with an N,O-acetal: A concise asymmetric synthesis of indolizidine alkaloid (-)-2-epi-lentiginosine. Tetrahedron 2012, 68, 1750–1755. [Google Scholar] [CrossRef]
- Behr, J.-B.; Mvondo Evina, C.; Phung, N.; Guillerm, G. Synthesis of (difluoromethyl)phosphonate azasugars designed as inhibitors for glycosyl transferases. J. Chem. Soc. Perkin Trans. 1 1997, 1597–1599. [Google Scholar] [CrossRef]
- Gautier-Lefebvre, I.; Behr, J.-B.; Guillerm, G.; Muzard, M. Iminosugars as glycosyltransferase inhibitors: Synthesis of polyhydroxypyrrolidines and their evaluation on chitin synthase activity. Eur. J. Med. Chem. 2005, 40, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.-B.; Guillerm, G. Synthesis of 6-deoxy-homoDMDP and its C(5)-epimer: Absolute stereochemistry of natural products from Hyacinthus orientalis. Tetrahedron Asymmetry 2002, 13, 111–113. [Google Scholar] [CrossRef]
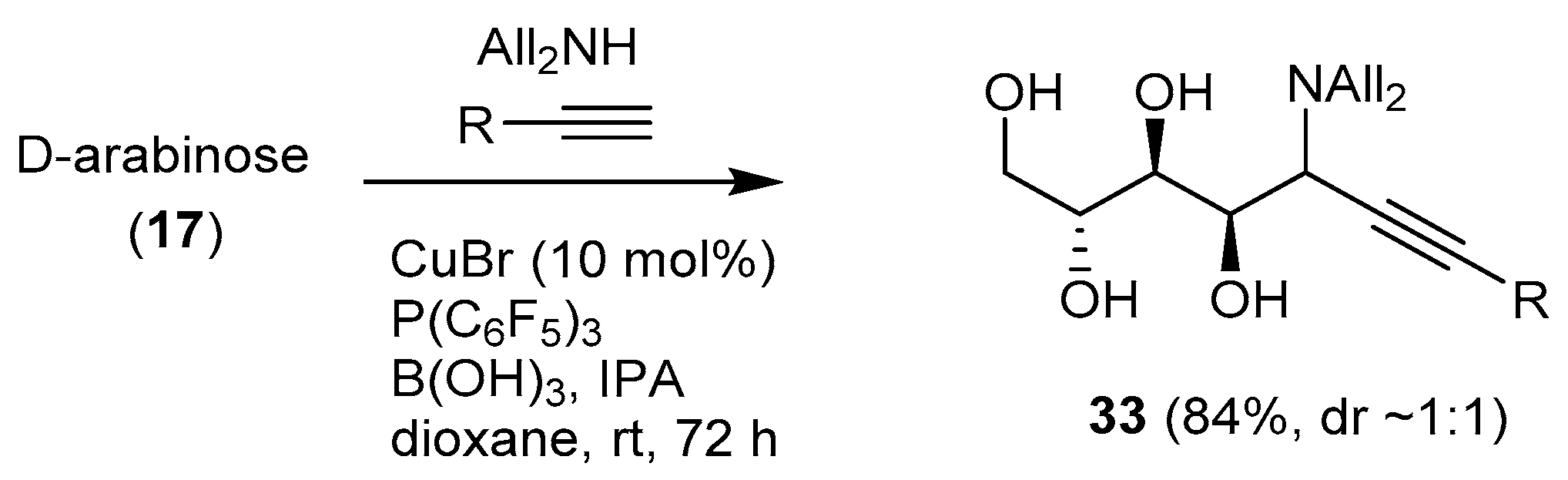
- Behr, J.-B.; Hottin, A.; Ndoye, A. Highly Selective Indium Mediated Allylation of Unprotected Pentosylamines. Org. Lett. 2012, 14, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.; Bisseret, P.; Constant, P.; Eustache, J. Synthesis of 2’,3’-dihydrosolanesyl analogues of β-d-arabinofuranosyl-1-monophosphoryldecaprenol with promising antimyco-bacterial activity. Tetrahedron Lett. 2007, 48, 153–157. [Google Scholar] [CrossRef]
- Schönemann, W.; Gallienne, E.; Compain, P.; Ikeda, K.; Asano, N.; Martin, O.R. Synthesis of new β-1-C-alkylated imino-l-iditols: A comparative study of their activity as β-glucocerebrosidase inhibitors. Bioorg. Med. Chem. 2010, 18, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Schönemann, W.; Gallienne, E.; Ikeda-Obatake, K.; Asano, N.; Nakagawa, S.; Kato, A.; Adachi, I.; Gorecki, M.; Frelek, J.; Martin, O.R. Glucosylceramide Mimics: Highly Potent GCase Inhibitors and Selective Pharmacological Chaperones for Mutations Associated with Types 1 and 2 Gaucher Disease. ChemMedChem 2013, 8, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.; Engo-Ilanga, F.; Cocaud, C.; Martin, O.R. En route to Novel Furanoside Mimics through Zinc-mediated Propargylation of N-benzyl Glycosylamines using Ultrasound Activation. Synlett 2015, 26, 187–192. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Siva Prasad, S.; Senthil Kumar, P.; Baskaran, S. A diversity oriented one-pot synthesis of novel iminosugar C-glycosides. Chem. Commun. 2014, 50, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
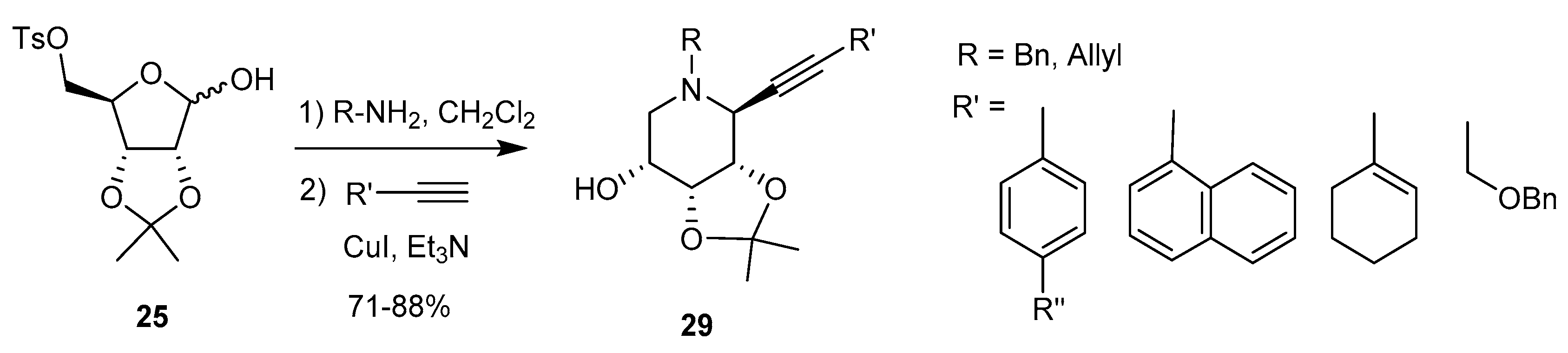
- Senthilkumar, S.; Siva Prasad, S.; Das, A.; Baskaran, S. One-pot Synthesis of Hydropho-bically Modified Iminosugar C-Alkynylglycosides: Facile Synthesis of Polyhydroxy Tetrahydroindolizidines. Chemistry 2015, 21, 15914–15918. [Google Scholar] [CrossRef] [PubMed]
- Siva Prasad, S.; Senthilkumar, S.; Srivastava, A.; Baskaran, S. Iminosugar C-Nitromethyl Glycosides and Divergent Synthesis of Bicyclic Iminosugars. Org. Lett. 2017, 19, 4403–4406. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, S.; Shimizu, Y.; Kanai, M. Catalytic Anomeric Aminoalkynylation of Unprotected Aldoses. Org. Lett. 2013, 15, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Naresh, A.; Marumudi, K.; Kunwar, A.C.; Rao, B.V. Palladium-Catalyzed Double Allylation of Sugar-Imines by Employing Tamaru–Kimura’s Protocol: Access to Unnatural Iminosugars. Org. Lett. 2017, 19, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Yoda, H.; Yamazaki, H.; Kawauchi, M.; Takabe, K. An Efficient Asymmetric Functionalization to Highly Substituted Pyrrolidines. Tetrahedron Asymmetry 1995, 6, 2669–2672. [Google Scholar] [CrossRef]
- Yoda, H.; Yamazaki, H.; Takabe, K. A Novel Synthesis of Enantiomerically Pure Antifungal Agent, (+)-Preussin. Tetrahedron Asymmetry 1996, 7, 373–374. [Google Scholar] [CrossRef]
- Dondoni, A.; Giovannini, P.P.; Perrone, D. New synthesis of Pyrrolidine Homoazasugars via Aminohomologation of Furanoses and Their Use for the Stereoselective Synthesis of Aza-C-disaccharides. J. Org. Chem. 2002, 67, 7203–7214. [Google Scholar] [CrossRef] [PubMed]
- Dondoni, A.; Perrone, D. A convenient synthesis of iminosugar-C-glycosides via organometallic addition to N-benzyl-N-glycosylhydroxylamines. Tetrahedron 2003, 59, 4261–4273. [Google Scholar] [CrossRef]
- Dondoni, A.; Nuzzi, A. Access to Piperidine Imino-C-glycosides via Stereoselective Thiazole-Based Aminohomologation of Pyranoses. J. Org. Chem. 2006, 71, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Cicchi, S.; Marradi, M.; Corsi, M.; Faggi, C.; Goti, A. Preparation of N-Glycosylhydroxylamines and Their Oxidation to Nitrones for the Enantioselective Synthesis of Isoxazolidines. Eur. J. Org. Chem. 2003, 4152–4160. [Google Scholar] [CrossRef]
- Dondoni, A.; Perrone, D. New entry to pyrrolidine homoazasugars: Conversion of d-arabinose into 2,5-anhydro-2,5-imino-d-glucitol via aminohomologation. Tetrahedron Lett. 1999, 40, 9375–9378. [Google Scholar] [CrossRef]
- Dondoni, A.; Perrone, D.; Rinaldi, M. Grignard Addition to Aldonitrones. Stereochemical Aspects and Application to the Synthesis of C2-Symmetric Diamino Alcohols and Diamino Diols. J. Org. Chem. 1998, 63, 9252–9264. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shimonishi, K.; Sakakura, Y.; Okada, S.; Asano, N.; Tanabe, Y. Facile and Practical Methods for the Sulfonylation of Alcohols Using Ts(Ms)Cl and Me2N(CH2)nNMe2 as a Key Base. Synthesis 1999, 1633–1636. [Google Scholar] [CrossRef]
- Argyropoulos, N.G.; Gkizis, P.; Coutouli-Argyropoulou, E. A convenient synthesis of new enantiomerically pure trihydroxypyrrolizidines using l-erythrose glycosylhydroxylamine as a masked acyclic chiral nitrone. Tetrahedron 2008, 64, 8752–8758. [Google Scholar] [CrossRef]
- Marradi, M.; Corsi, M.; Cicchi, S.; Bonanni, M.; Cardona, F.; Goti, A. N-Glycosylhydroxylamines as Masked Polyhydroxylated Chiral Nitrones in Cycloaddition Reactions: An Access to Pyrrolizidines. Heterocycles 2009, 79, 883–896. [Google Scholar] [CrossRef]
- Malinowski, M.; Rowicki, T.; Guzik, P.; Gryszel, M.; Łapczyński, S.; Wielechowska, M.; Czerwińska, K.; Madura, I.; Sas, W. [1,4]-sigmatropic rearrangement of chiral nitrones and their utilization in the synthesis of new iminosugars. Org. Biomol. Chem. 2016, 14, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Kobayashi, S. Lewis Acid-Catalyzed Ring-Opening Reactions of Semicyclic N,O-Acetals. Org. Lett. 2001, 3, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Hagio, H.; Hirabayashi, R.; Kobayashi, S. Lewis Acid-Catalyzed Ring-Opening Reactions of Semicyclic N,O-Acetals Possessing an Exocyclic Nitrogen Atom: Mechanistic Aspect and Application to Piperidine Alkaloid Synthesis. J. Am. Chem. Soc. 2001, 123, 12510–12517. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Hagio, H.; Kobayashi, S. Lewis Acid Catalyzed Ring-Opening Reactions of Sugar-Derived Semicyclic N,O-Acetals. Helv. Chim. Acta 2002, 85, 3678–3691. [Google Scholar] [CrossRef]
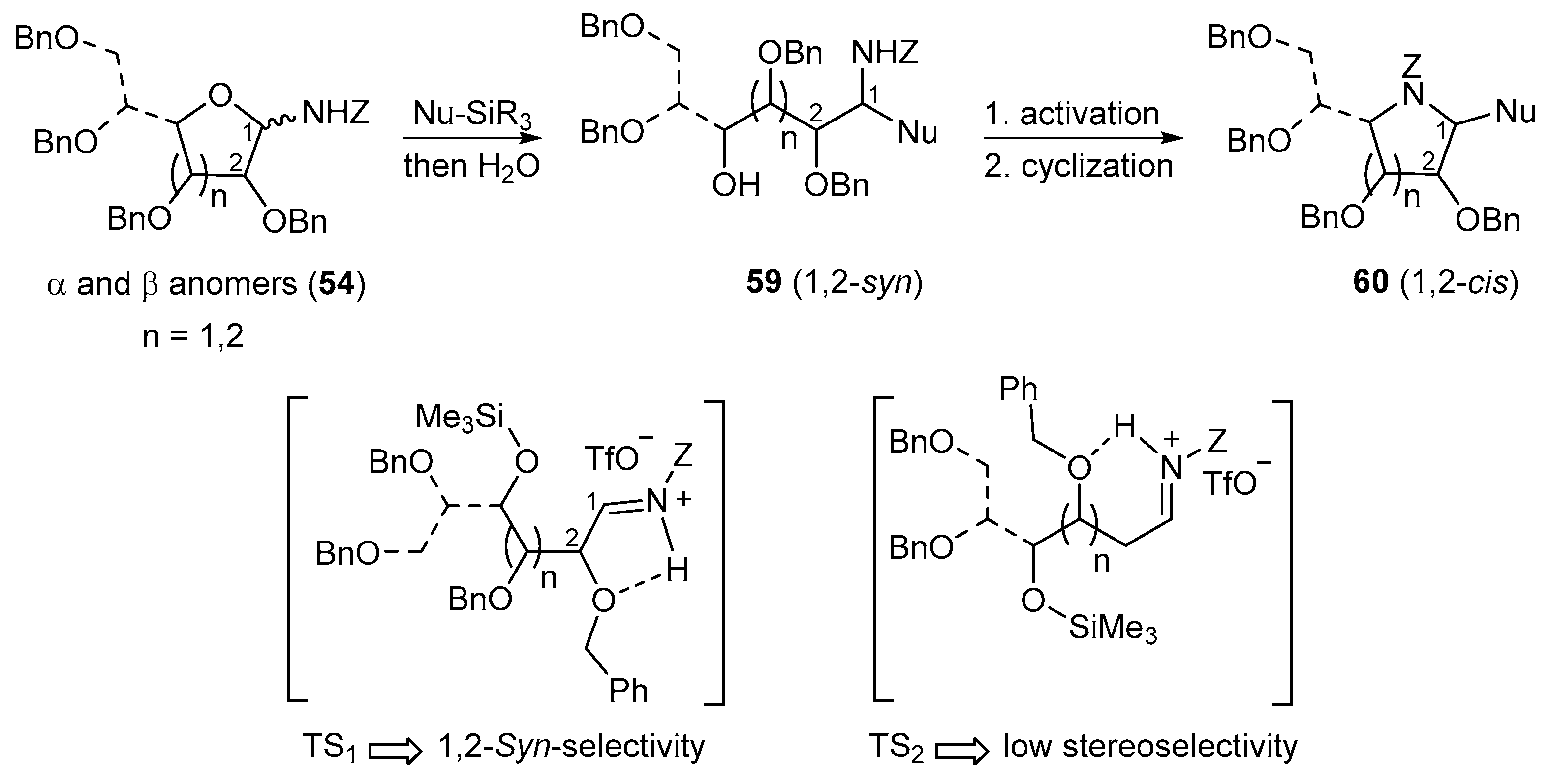
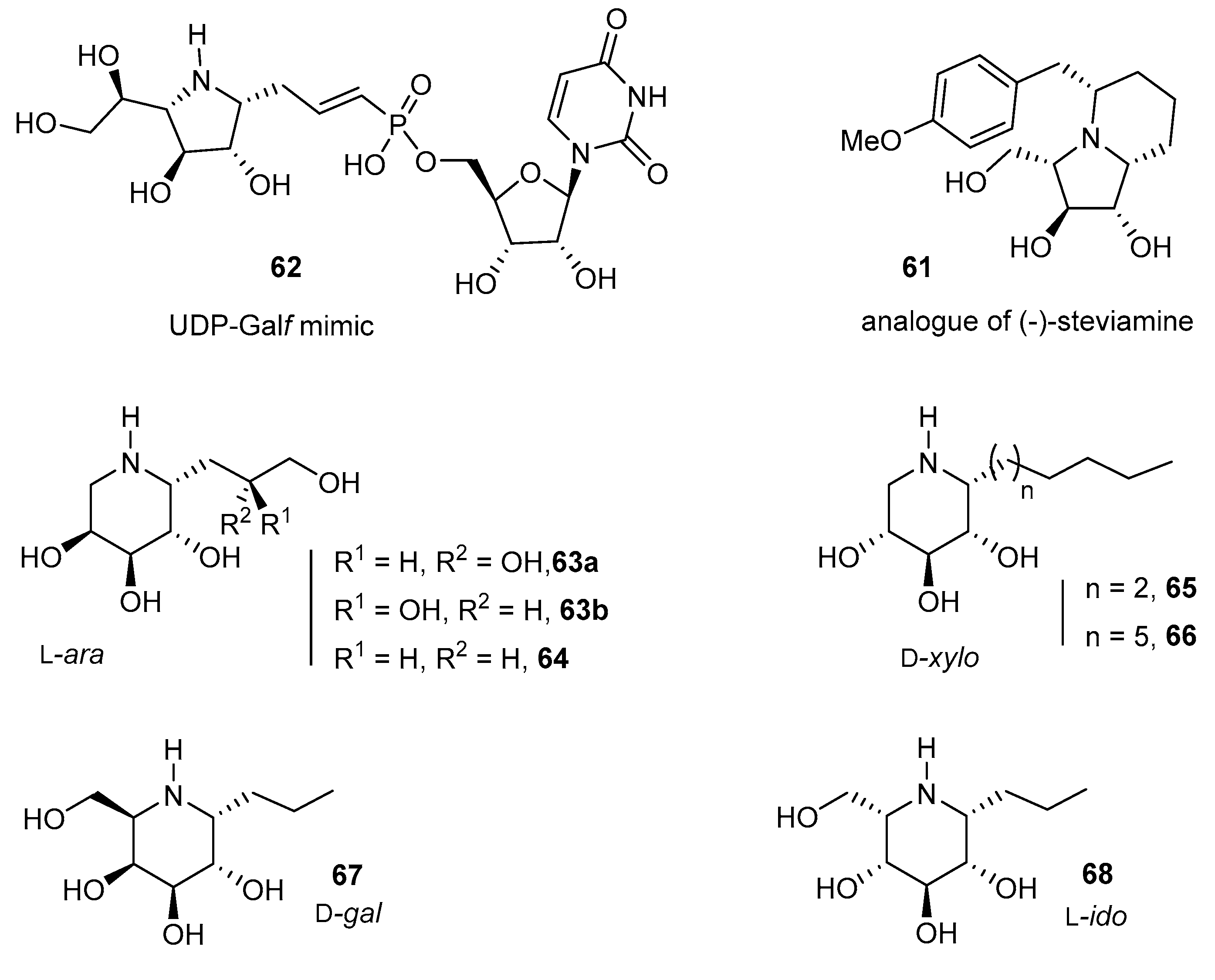
- Liautard, V.; Desvergnes, V.; Martin, O.R. Stereoselective Synthesis of α-C-Substituted 1,4-Dideoxy-1,4-imino-d-galactitols. Toward Original UDP-Galf Mimics via Cross-Metathesis. Org. Lett. 2006, 8, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Liautard, V.; Desvergnes, V.; Itoh, K.; Liu, H.-W.; Martin, O.R. Convergent and Stereoselective Synthesis of Iminosugar-Containing Galf and UDP-Galf Mimicks: Evaluation as Inhibitors of UDP-Gal Mutase. J. Org. Chem. 2008, 73, 3103–3115. [Google Scholar] [CrossRef] [PubMed]
- Biela, A.; Oulaïdi, F.; Gallienne, E.; Górecki, M.; Frelek, J.; Martin, O.R. An improved methodology for the synthesis of 1-C-allyl imino-d-xylitol and -l-arabinitol and their rapid functionalization. Tetrahedron 2013, 69, 3348–3354. [Google Scholar] [CrossRef]
- Liautard, V.; Pillard, C.; Desvergnes, V.; Martin, O.R. One-step synthesis of N-protected glycosylamines from sugar hemiacetals. Carbohydr. Res. 2008, 343, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Biela-Banaś, A.; Gallienne, E.; Front, S.; Martin, O.R. Stereoselective synthesis of 1-C-alkyl iminogalactitol derivatives, potential chaperones for galactosidase-linked LSDs: A real challenge. Tetrahedron Lett. 2014, 55, 838–841. [Google Scholar] [CrossRef]
- Chronowska, A.; Gallienne, E.; Nicolas, C.; Kato, A.; Adachi, I.; Martin, O.R. An expeditious synthesis of an analogue of (–)-steviamine by way of the 1,3-dipolar cycloaddition of a nitrile oxide with a 1-C-allyl iminosugar. Tetrahedron Lett. 2011, 52, 6399–6402. [Google Scholar] [CrossRef]
- Rawal, G. K.; Kumar, A.; Tawar, U.; Vankar, Y. D. New Method for Chloroamidation of Olefins. Application in the Synthesis of N-Glycopeptides and Anticancer Agents. Org. Lett. 2007, 9, 5171–5174. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Reddy, B.G.; Vankar, Y.D. Efficient and Stereoselective Syntheses of d- and l-Fagomines and Their Analogues. Eur. J. Org. Chem. 2009, 160–169. [Google Scholar] [CrossRef]
- Kumari, N.; Vankar, Y.D. Synthesis and glycosidase-inhibitory activity of novel polyhydroxylated quinolizidines derived from d-glycals. Org. Biomol. Chem. 2009, 7, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Cocaud, C.; Nicolas, C.; Bayle, A.; Poisson, T.; Pannecoucke, X.; Martin, O.R. Synthesis and Reactivity of N-tert-Butanesulfinyl Glycosylamines. Eur. J. Org. Chem. 2015, 4330–4334. [Google Scholar] [CrossRef]
- Cocaud, C.; Nicolas, C.; Désiré, J.; Martin, O.R. One-Step Preparation of Protected N-tert-Butanesulfinyl d-ribo and d-xylo Furanosylamines from Related Sugar Hemiacetals. In Carbohydrate Chemistry: Proven Synthetic Methods; Vogel, C., Murphy, P., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Volume 4, pp. 45–53. ISBN 978-1-4987-2691-7. [Google Scholar]
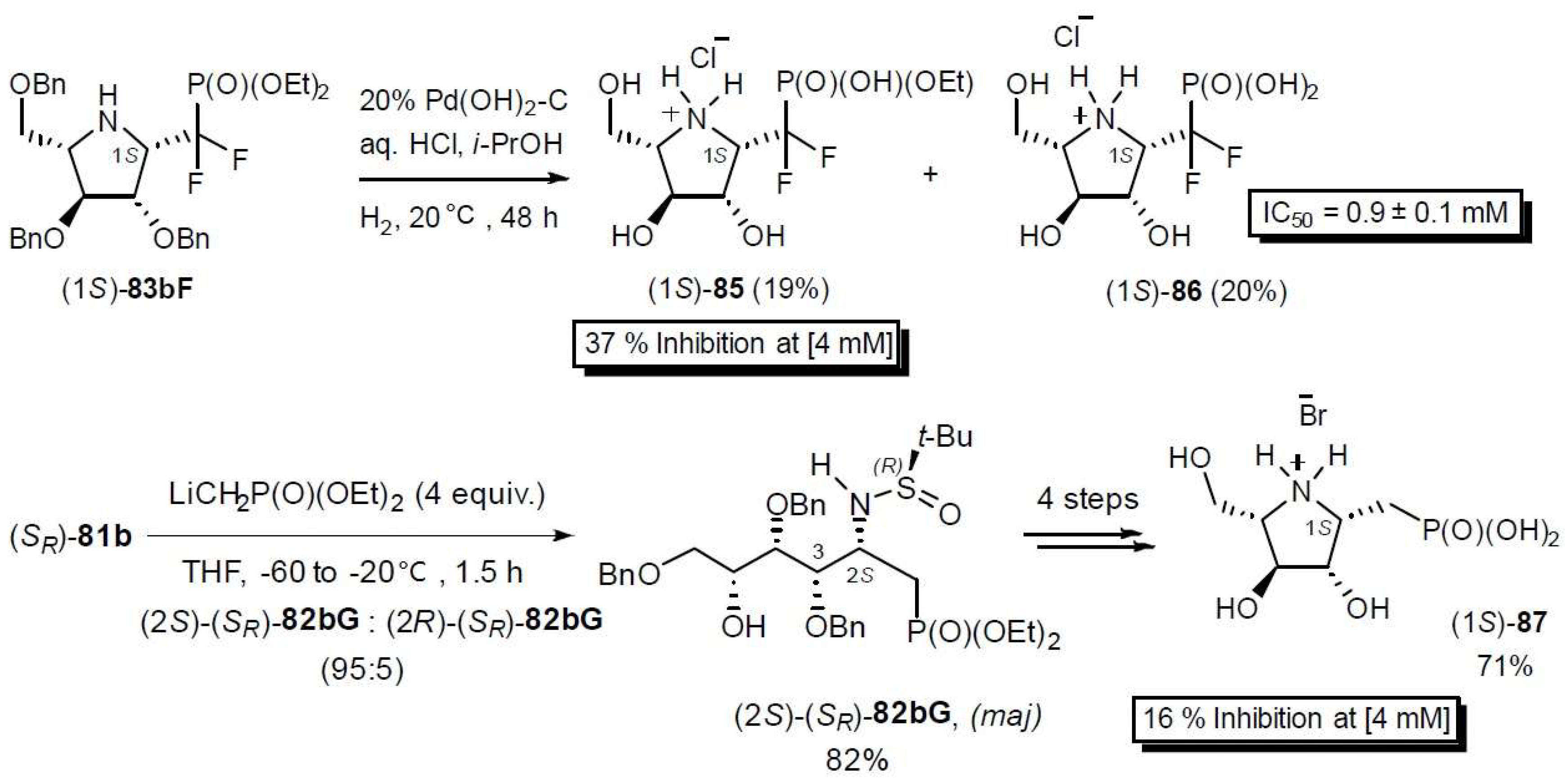
- Cocaud, C.; Nicolas, C.; Poisson, T.; Pannecoucke, X.; Legault, C.Y.; Martin, O.R. Tunable Approach for the Stereoselective Synthesis of 1-C-Diethylphosphono(difluoromethyl) Iminosugars as Glycosyl Phosphate Mimics. J. Org. Chem. 2017, 82, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
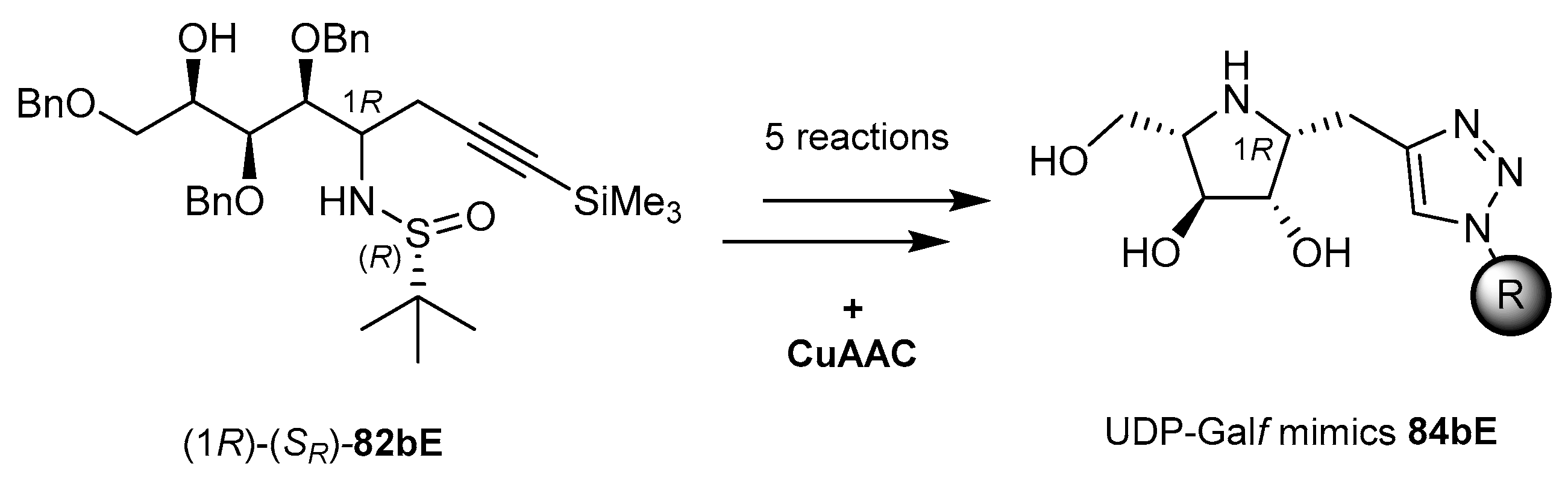
- Cocaud, C.; Maujoin, A.; Zheng, R.B.; Lowary, T.L.; Rodrigues, N.; Percina, N.; Chartier, A.; Buron, F.; Routier, S.; Nicolas, C.; et al. Triazole-Linked Iminosugars and Aromatic Systems as Simplified UDP-Galf Mimics: Synthesis and Preliminary Evaluation as Galf-Transferase Inhibitors. Eur. J. Org. Chem. 2017, 2017, 6192–6201. [Google Scholar] [CrossRef]
- Cocaud, C.; Zheng, R.B.; Lowary, T.L.; Poisson, T.; Pannecoucke, X.; Nicolas, C.; Martin, O.R. 1-C-phosphonomethyl- and 1-C-difluorophosphonomethyl-1,4-imino-l-arabinitols as Galf transferase inhibitors: A comparison. Carbohydr. Res. 2018, 461, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Poulin, M.B.; Lowary, T.L. Chemical Insight into the Mechanism and Specificity of GlfT2, a Bifunctional Galactofuranosyltransferase from Mycobacteria. J. Org. Chem. 2016, 81, 8123–8130. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Jackson, M.; Ma, Y.; McNeil, M. Cell Wall Core Galactofuran Synthesis Is Essential for Growth of Mycobacteria. J. Bacteriol. 2001, 183, 3991–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Mikusova, K.; Robuck, K.; Scherman, M.; Brennan, P.; McNeil, M. Recognition of Multiple Effects of Ethambutol on Metabolism of Mycobacterial Cell Envelope. Antimicrob. Agents Chemother. 1995, 39, 694–701. [Google Scholar] [CrossRef] [PubMed]



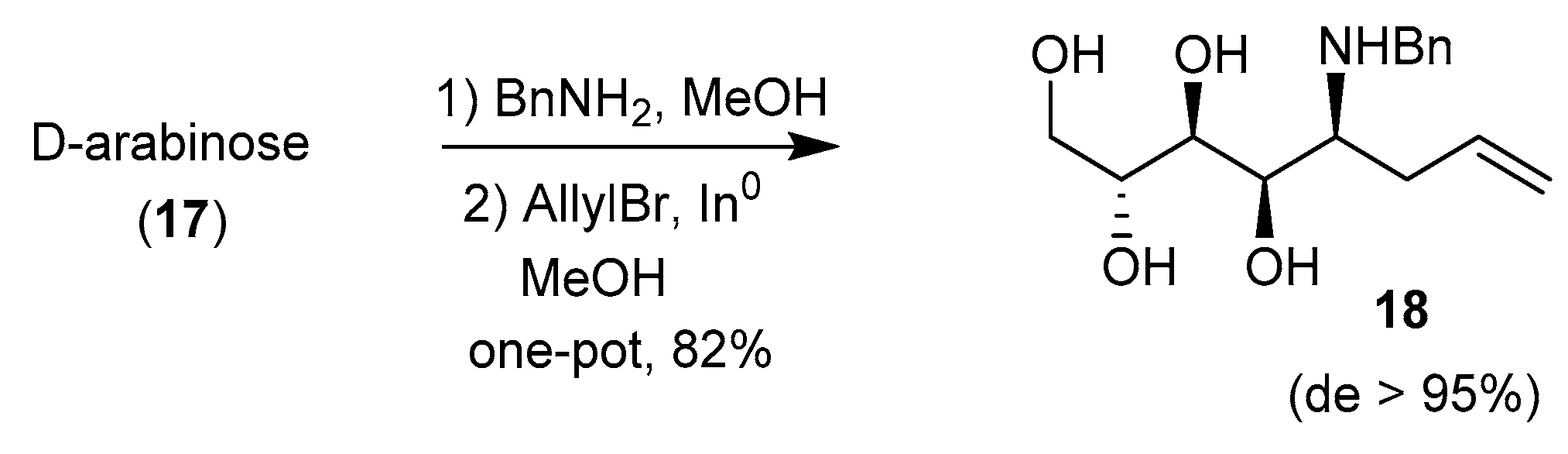














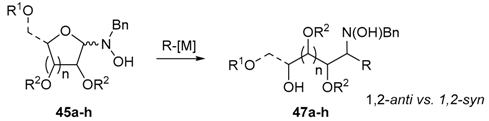 | ||||
|---|---|---|---|---|
| Entry | Hydroxylamine | R-[M] | Yield (%) [a] | anti/syn [b] |
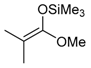
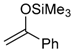
| 1 | 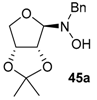 | 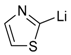 | 65 | 98/2 |
| 2 | 45a |  | 72 | 70/30 |
| 3 | 45a | 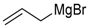 | 95 | 96/4 |
| 4 | 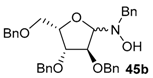 |  | 72 | 80/20 |
| 5 | 45b | 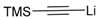 | 82 | 90/10 |
| 6 | 45b | 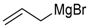 | 80 | 85/15 |
| 7 |  |  | 72 [c] | ND [d] |
| 8 | 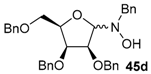 | 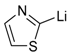 | 52 [c] | 70/30 |
| 9 |  | 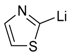 | 75 | 90/10 |
| 10 | 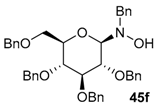 |  | 80 | 25/75 |
| 11 | 45f |  | 85 | 85/15 |
| 12 | 45f | 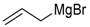 | 85 | 50/50 |
| 13 | 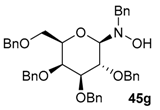 |  | 77 | 33/67 |
| 14 | 45g | 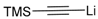 | 85 | 75/25 |
| 15 | 45g | 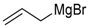 | 90 | 50/50 |
| 16 | 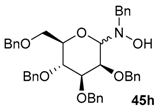 |  | 82 | 60/40 |
 | ||||
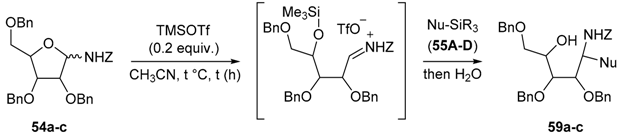 | |||
 |  |  |  |
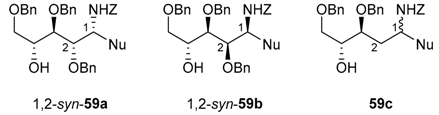
 55A | −40 °C, 12 h [a] 59aA (quant.) [b,d] syn:anti 93:7 | −40 °C, 21 h [a] 59bA (62%) [b,d] syn:anti 91:9 | - |
| Me2ClSiH 55B | 0 °C, 6 h [c] 59aB (76%) [b,d] | 0 °C, 9 h [c] 59bB (59%) [b,d] | - |
 55C | −40 °C, 16 h [a] 59aC (70%) [b,d] syn:anti 93:7 | −40 °C, 37 h [a] 59bC (77%) [b,d] syn:anti 93:7 | −40 °C, 9 h [a] 59cC (80%) [b,d] syn:anti 50:50 |
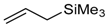 55D | 0 °C, 23 h [a] 59aD (0%) [b,d] | −30 °C, 39 h [a] 59bD (64%) [b,d] syn:anti > 94:6 | - |
 | |||
 | |||||
|---|---|---|---|---|---|
| Entry | Reagent (equiv.) | Auxiliary (SR or SS) | Dr [a] | Yield (%) [b,c] | Rf [d] |
| 1 | PhMgBr (3.5) | SR | 7:3 | 69 | 0.3 |
| 2 | PhMgBr (3.5) | SR | 10:0 [e] | 30 | – |
| 3 | PhMgBr (3.5) | SR | 85:15 [e,f] | 60 | – |
| 4 | vinMgBr (6) | SR | 8:2 | 69 | 0.5/0.3 |
| 5 | n-hexMgBr (6) | SR | 6:4 | 45 | 0.5 |
| 6 | BnMgCl (3.5) | SR | 7:3 | 83 | 0.6/0.55 |
| 7 | BnMgCl (3.5) | SR | 9:1 [e] | 95 | |
| 8 | PhMgBr (3.5) | SS | 7:3 | 57 | 0.5/0.2 |
| 9 | vinMgBr (6) | SS | 8:2 | 62 | 0.3/0.1 |
| 10 | n-hexMgBr (6) | SS | 6:4 | 40 | 0.5/0.4 |
| 11 | BnMgCl (3.5) | SS | ~85:15 | 94 | 0.6/0.5 |
| 12 | BnMgCl (3.5) | SS | 97:3 [e] | 83 | – |
| 13 | allMgBr (3.5) | SS | 5:5 | 83 | 0.6/0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolas, C.; Martin, O.R. Glycoside Mimics from Glycosylamines: Recent Progress. Molecules 2018, 23, 1612. https://doi.org/10.3390/molecules23071612
Nicolas C, Martin OR. Glycoside Mimics from Glycosylamines: Recent Progress. Molecules. 2018; 23(7):1612. https://doi.org/10.3390/molecules23071612
Chicago/Turabian StyleNicolas, Cyril, and Olivier R. Martin. 2018. "Glycoside Mimics from Glycosylamines: Recent Progress" Molecules 23, no. 7: 1612. https://doi.org/10.3390/molecules23071612














