Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy
Abstract
:1. Introduction
2. Recent Progress of Photodynamic Therapy
- (1)
- (2)
- Low lymph drainage characteristic of tumor area. The decrease in the drainage function of the lymphatic system contributes to the fact that photosensitizers are slowly excreted from the tumor site, which leads to their local accumulation [68].
- (3)
- High speed of proliferation in the tumor, in which there is a high level of expression of low-density lipoprotein receptors, binding a large number of hydrophobic molecules of photosensitizer [69].
- (4)
- Lower pH value of the tumor than in healthy tissues. The main reason for strengthening the accumulation of photosensitizers in the acidification of the environment is to increase the lipophilicity of the drug, if protonated [70].
- (5)
- Abnormal structure of the tumor stroma, characterized by increased intercellular space and increased production of collagen, which binds porphyrins [71].
- (6)
- Large number of macrophages in tumor tissue, which are effective traps for hydrophobic photosensitizers [72].
3. Applications of Metal-Based Nanoparticles in PDT
3.1. Gold Nanoparticles
3.2. Silver Nanoparticles
3.3. Copper-Based Nanoparticles
3.4. Magnetic Nanoparticles
3.5. Metal-Organic Frameworks in PDT
4. Potential Toxicity of Metal-Based Nanoparticles
4.1. Gold Nanoparticles
4.2. Silver Nanoparticles
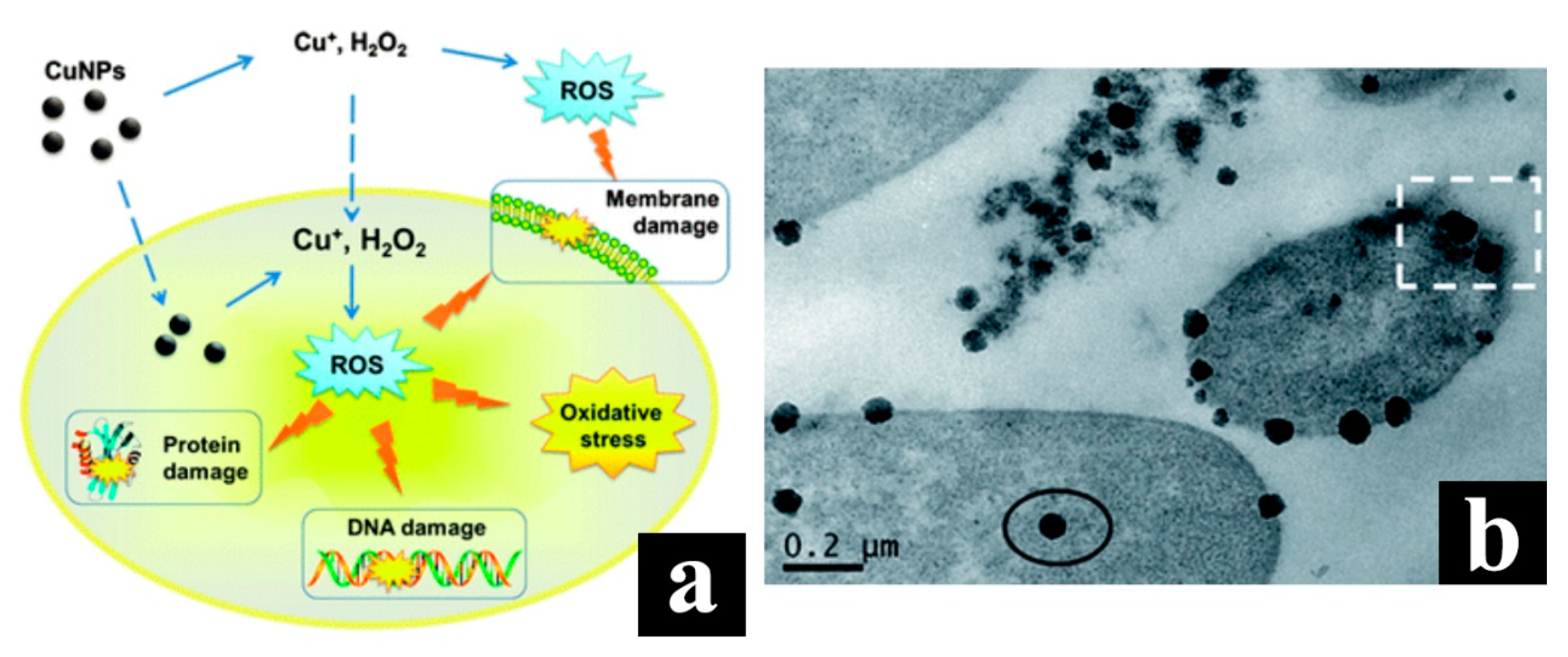
4.3. Copper Nanoparticles
5. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic therapy and diagnosis: Principles and comparative aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.H.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Mallidi, S.; Liu, J.; Chiang, C.T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer. Cancer Res. 2015, 76, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Xu, X.; Cai, Y.; Zou, L.; Shuai, X. Perfluorohexane-cored nanodroplets for stimulations-responsive ultrasonography and O2-potentiated photodynamic therapy. Biomaterials 2018, 175, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kachynski, A.V.; Pliss, A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic therapy by in situ nonlinear photon conversion. Nat. Photonics 2014, 8, 455–461. [Google Scholar] [CrossRef]
- Yu, M.; Guo, F.; Wang, J.; Tan, F.; Li, N. A pH-Driven and photoresponsive nanocarrier: Remotely-controlled by near-infrared light for stepwise antitumor treatment. Biomaterials 2016, 79, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Hanaoka, K.; Fujisawa, T.; Takeuchi, S.; Komatsu, T.; Ueno, T.; Terai, T.; Tahara, T.; Nagano, T.; Urano, Y. Development of an Azo-Based Photosensitizer Activated under Mild Hypoxia for Photodynamic Therapy. J. Am. Chem. Soc. 2017, 139, 13713–13719. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.P.; da Silva, F.C.; Nader, S.A.; Meira, G.A.; Viana, M.S. In vitro effectiveness of antimicrobial photodynamic therapy (APDT) using a 660 nm laser and malachite green dye in Staphylococcus aureus biofilms arranged on compact and cancellous bone specimens. Lasers Med. Sci. 2014, 29, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An Injectable Self-Assembling Collagen-Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Miller, J.; Yuan, M.; Liu, J.F.; Busch, T.M.; Tsourkas, A.; Cheng, Z. Improved Photodynamic Therapy Efficacy of Protoporphyrin IX-Loaded Polymeric Micelles Using Erlotinib Pretreatment. Biomacromolecules 2017, 18, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core–Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, É.P.; Campos, L.; Pereira, F.S.; Magliano, G.C.; Benites, B.M.; Arana-Chavez, V.E.; Ballester, R.Y.; Simões, A. Clinical, biochemical and histological study of the effect of antimicrobial photodynamic therapy on oral mucositis induced by 5-fluorouracil in hamsters. Photodiag. Photodyn. Ther. 2015, 12, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Chang, K.; Sun, K.; Tang, Y.; Cui, N.; Wang, Y.; Qin, W.; Xu, H.; Wu, C. Amplified Singlet Oxygen Generation in Semiconductor Polymer Dots for Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 8, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Marcus, S.L.; Pottier, R.H. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Mechanisms and clinical results. J. Clin. Laser Med. Surg. 1996, 14, 289–304. [Google Scholar] [PubMed]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal–Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.N.; Keskin Tunc, S.; Erten, R.; Usumez, A. Clinical and histological evaluation of the efficacy of antimicrobial photodynamic therapy used in addition to antibiotic therapy in pericoronitis treatment. Photodiagn. Photodyn. Ther. 2018, 21, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kapoor, U.; Juneja, M.; Nagpal, A. Halitosis: Current concepts on etiology, diagnosis and management. Eur. J. Dent. 2016, 10, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.H. History of Photodynamic Therapy. In Photodynamic Therapy; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–22. [Google Scholar] [CrossRef]
- Allison, R.; Moghissi, K.; Downie, G.; Dixon, K. Photodynamic therapy (PDT) for lung cancer. Photodiagn. Photodyn. Ther. 2011, 8, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T. Photodynamic therapy as a unique enabler of spatiotemporally synchronized cancer combination therapies. Photodiagn. Photodyn. Ther. 2017, 17, A33–A34. [Google Scholar] [CrossRef]
- Kaya, E.N.; Köksoy, B.; Yeşilot, S.; Durmuş, M. The effects of axially BODIPY substitution on photodynamic therapy of cancer properties of silicon (IV) phthalocyanine. Photodiagn. Photodyn. Ther. 2017, 17, A56. [Google Scholar] [CrossRef]
- Raab, O. Uber die Wirkung fluorescirender Stoffe auf Infusorien. Ztg. Biol. 1900, 39, 524–546. [Google Scholar]
- Hausmann, W. Über die sensibilisierende Wirkung des Hämatoporphyrins. Biochem. Z. 1911, 30, 276–316. [Google Scholar]
- Meyer-Betz, F. Untersuchungen über die biologische (photodynamische) Wirkung des Hämatoporphyrins und anderer Derivate des Blut-und Gallenfarbstoffs. Dtsch. Arch. Klin. Med. 1913, 112, 476–503. [Google Scholar]
- Policard, A. Etude sur les aspects offerts par des tumeurs experimentales examinees a la lumiere de Wood. C. R. Soc. Biol. 1924, 91, 1423–1424. [Google Scholar]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Lam, S.; Müller, N.L.; Miller, R.R.; Kostashuk, E.C.; Szasz, I.J.; Leriche, J.C.; Lee-Chuy, E. Predicting the response of obstructive endobronchial tumors to photodynamic therapy. Cancer 1986, 58, 2298–2306. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; MacRobert, A.; Mosse, C.; Periera, B.; Bown, S.; Keshtgar, M. Photodynamic therapy: Inception to application in breast cancer. Breast 2017, 31, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Peng, J.; Tan, L.; Wu, J.; Shi, K.; Qu, Y.; Wei, X.; Qian, Z. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified Docetaxel/IR820 Co-loaded micelles. Biomaterials 2016, 106, 119–133. [Google Scholar] [CrossRef] [PubMed]
- García Calavia, P.; Chambrier, I.; Cook, M.J.; Haines, A.H.; Field, R.A.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J. Colloid Interface Sci. 2018, 512, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jiang, Q.; Feng, D.; Mao, L.; Zhou, H.-C. Size-Controlled Synthesis of Porphyrinic Metal–Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Lei, J.; Shen, H.; Ju, H. Multifunctional Metal–Organic Framework Nanoprobe for Cathepsin B-Activated Cancer Cell Imaging and Chemo-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Weichselbaum, R.R.; Lin, W. Chlorin-Based Nanoscale Metal–Organic Framework Systemically Rejects Colorectal Cancers via Synergistic Photodynamic Therapy and Checkpoint Blockade Immunotherapy. J. Am. Chem. Soc. 2016, 138, 12502–12510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golding, J.P.; Kemp-Symonds, J.G.; Dobson, J.M. Glycolysis inhibition improves photodynamic therapy response rates for equine sarcoids. Vet. Comp. Oncol. 2017, 15, 1543–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsella, T.J.; Colussi, V.C.; Oleinick, N.L.; Sibata, C.H. Photodynamic therapy in oncology. Expert Opin. Pharmacother. 2005, 2, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Grossman, C.; Carter, S.; Czupryna, J.; Wang, L.; Putt, M.; Busch, T. Fluence Rate Differences in Photodynamic Therapy Efficacy and Activation of Epidermal Growth Factor Receptor after Treatment of the Tumor-Involved Murine Thoracic Cavity. Int. J. Mol. Sci. 2016, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.; Wu, J.; Jiang, C.; Shen, J.; Cooper, M.A.; Zheng, X.; Liu, Y.; Yang, Z.; Wu, D. Biomimetic Moth-eye Nanofabrication: Enhanced Antireflection with Superior Self-cleaning Characteristic. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, Y.; Yang, Z.; Shen, J.; Cabrera, E.; Lertola, M.J.; Yang, W.; Zhang, D.; Benatar, A.; Castro, J.M.; et al. Highly Stretchable and Ultrathin Nanopaper Composites for Epidermal Strain Sensors. Nanotechnology 2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2016, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yin, H.; Jabed, M.A.; Hetu, M.; Wang, C.; Monro, S.; Zhu, X.; Kilina, S.; McFarland, S.A.; Sun, W. π-Expansive Heteroleptic Ruthenium(II) Complexes as Reverse Saturable Absorbers and Photosensitizers for Photodynamic Therapy. Inorg. Chem. 2017, 56, 3245–3259. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.D.; Paulo, T.F.; Gehlen, M.H.; Ando, R.A.; Lopes, L.G.F.; Gondim, A.C.S.; Vasconcelos, M.A.; Teixeira, E.H.; Sousa, E.H.S.; de Carvalho, I.M.M. Aryl-Substituted Ruthenium(II) Complexes: A Strategy for Enhanced Photocleavage and Efficient DNA Binding. Inorg. Chem. 2017, 56, 9084–9096. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.C.; Weersink, R.A. Photodynamic therapy for periodontal disease. In Opto-Canada: SPIE Regional Meeting on Optoelectronics, Photonics, and Imaging; SPIE: Bellingham, WA, USA, 2017; p. 108. [Google Scholar] [CrossRef]
- Spring, B.Q.; Rizvi, I.; Xu, N.; Hasan, T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem. Photobiol. Sci. 2015, 14, 1476–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Mizushima, N. Autophagy and human diseases. Cell Res. 2013, 24, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, J.; Cunha, L.D.; Park, S.; Yang, M.; Lu, Q.; Orchard, R.; Li, Q.-Z.; Yan, M.; Janke, L.; Guy, C.; et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. TIMELINE: Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2016, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.; Tsubone, T.; Pavani, C.; Baptista, M. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, K.M.; Foit, L.; Angeloni, N.L.; Giles, F.J.; Gordon, L.I.; Thaxton, C.S. Synthetic High-Density Lipoprotein-Like Nanoparticles as Cancer Therapy. Cancer Treat Res. 2015, 166, 129–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xodo, L.E.; Cogoi, S.; Rapozzi, V. Photosensitizers binding to nucleic acids as anticancer agents. Future Med. Chem. 2016, 8, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Svenmarker, P.; Axelsson, J.; Gräfe, S.; Kyriazi, M.; Bendsoe, N.; Andersson-Engels, S.; Svanberg, K. Pharmacokinetic and biodistribution study following systemic administration of Fospeg®—A Pegylated liposomal mTHPC formulation in a murine model. J. Biophotonics 2015, 8, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.O.; Nabeshima, C.T.; Bellini, M.H.; Schor, N.; Vieira, N.D.; Courrol, L.C. Study of ProtoPorphyrin IX Elimination by Body Excreta: A new Noninvasive Cancer Diagnostic Method? J. Fluoresc. 2012, 23, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, T.; Hansen, C.G.; Guan, K.-L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Xie, J.; Zhu, J.; Kang, C.; Chiang, C.; Wang, X.; Wang, X.; Kuang, T.; Chen, F.; Chen, Z.; et al. Functional exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo evaluation. J. Controll. Release 2016, 243, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Davis, M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015, 14, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, Z.; Hu, Y.; Rao, Z.; Wu, W.; Yang, Z. Nanocrystals: The preparation, precise control, and application toward the pharmaceutics and foods industry. Curr. Pharm. Des. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Yankovsky, I.; Millard, M.; Lamy, L.; Lassalle, H.-P.; Wiehe, A.; Zorin, V.; Bezdetnaya, L. The alteration of temoporfin distribution in multicellular tumor spheroids by β-cyclodextrins. Int. J. Pharm. 2017, 529, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Controll. Release 2012, 161, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormond, A.; Freeman, H. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehraban, N.; Freeman, H. Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials 2015, 8, 4421–4456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessel, D.; Conley, M.; Vicente, M.G.H.; Reiners, J.J. Studies on the Subcellular Localization of the Porphycene CPO. Photochem. Photobiol. 2005, 81, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Marchal, S.; François, A.; Dumas, D.; Guillemin, F.; Bezdetnaya, L. Relationship between subcellular localisation of Foscan® and caspase activation in photosensitised MCF-7 cells. Br. J. Cancer 2007, 96, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Rui, L.-L.; Cao, H.-L.; Xue, Y.-D.; Liu, L.-C.; Xu, L.; Gao, Y.; Zhang, W.-A. Functional organic nanoparticles for photodynamic therapy. Chin. Chem. Lett. 2016, 27, 1412–1420. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Y.; Chen, Q.; Xing, D. In Vivo Near-Infrared Photodynamic Therapy Based on Targeted Upconversion Nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Thompson, C.B. Cellular Metabolism and Disease: What Do Metabolic Outliers Teach Us? Cell 2012, 148, 1132–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Hsu, Y.-C.; Li, L.-B.; Wang, L.-W.; Song, X.-D.; Yow, C.M.N.; Lei, X.; Musani, A.I.; Luo, R.-C.; Day, B.J. Photodynamic therapy of cancer—Challenges of multidrug resistance. J. Innov. Opt. Health Sci. 2015, 08, 1530002. [Google Scholar] [CrossRef]
- Debele, T.; Peng, S.; Tsai, H.-C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Liu, D.; Lin, W. Self-Assembled Core–Shell Nanoparticles for Combined Chemotherapy and Photodynamic Therapy of Resistant Head and Neck Cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The potential of photodynamic therapy (PDT)—Experimental investigations and clinical use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Z.; Zhang, A.; Hu, J.; Wang, X.; Yang, Z. Electrospun nanofibers for cancer diagnosis and therapy. Biomater. Sci. 2016, 4, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, Z.; Teng, L. Nanomedicine based on nucleic acids: Pharmacokinetic and pharmacodynamic perspectives. Curr. Pharm. Biotechnol. 2014, 15, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, B.; Zhu, J.; Huang, X.; Xie, J.; Xu, S.; Yang, X.; Wang, X.; Yung, B.C.; Lee, L.J.; et al. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale 2014, 6, 9742–9751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, A.; Wang, X.; Zhu, J.; Fan, Y.; Yu, H.; Yang, Z. The Advances of Carbon Nanotubes in Cancer Diagnostics and Therapeutics. J. Nanomater. 2017, 2017, 3418932. [Google Scholar] [CrossRef]
- Lee, L.J.; Yang, Z.; Rahman, M.; Ma, J.; Kwak, K.J.; McElroy, J.; Shilo, K.; Goparaju, C.; Yu, L.; Rom, W.; et al. Extracellular mRNA Detected by Tethered Lipoplex Nanoparticle Biochip for Lung Adenocarcinoma Detection. Am. J. Respir. Crit. Care Med. 2016, 193, 1431–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Cong, M.; Hu, J.; Yang, Z.; Chen, Z. Preparation of Functionalized TiO2 Nanotube Arrays and Their Applications. Sci. Adv. Mater. 2016, 8, 1231–1241. [Google Scholar] [CrossRef]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic Acid Based Nanocomposites: Promising Safe and Biodegradable Materials in Biomedical Field. Int. J. Polym. Sci. 2016, 2016, 68691541. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, L.; Chiang, C.L.; Lee, L.J. Micro-/nano-electroporation for active gene delivery. Curr. Pharm. Des. 2015, 21, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chang, L.; Li, W.; Xie, J. Novel biomaterials and biotechnology for nanomedicine. Eur. J. Med. Res. 2015, 1, 1–2. [Google Scholar] [CrossRef]
- Chouikrat, R.; Seve, A.; Vanderesse, R.; Benachour, H.; Barberi-Heyob, M.; Richeter, S.; Raehm, L.; Durand, J.O.; Verelst, M.; Frochot, C. Non Polymeric Nanoparticles for Photodynamic Therapy Applications: Recent Developments. Curr. Med. Chem. 2012, 19, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Sortino, S. Photoactivated nanomaterials for biomedical release applications. J. Mater. Chem. 2012, 22, 301–318. [Google Scholar] [CrossRef]
- Xie, J.; Teng, L.; Yang, Z.; Zhou, C.; Liu, Y.; Yung, B.C.; Lee, R.J. A polyethylenimine-linoleic acid conjugate for antisense oligonucleotide delivery. BioMed Res. Int. 2013, 2013, 710502. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, A.; Yang, Z.; Wang, X.; Chang, L.; Chen, Z.; James Lee, L. Application of DODMA and Derivatives in Cationic Nanocarriers for Gene Delivery. Curr. Org. Chem. 2016, 20, 1813–1819. [Google Scholar] [CrossRef]
- Calixto, G.; Bernegossi, J.; de Freitas, L.; Fontana, C.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Svenskaya, Y.; Parakhonskiy, B.; Haase, A.; Atkin, V.; Lukyanets, E.; Gorin, D.; Antolini, R. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys. Chem. 2013, 182, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Colombeau, L.; Acherar, S.; Baros, F.; Arnoux, P.; Gazzali, A.M.; Zaghdoudi, K.; Toussaint, M.; Vanderesse, R.; Frochot, C. Inorganic Nanoparticles for Photodynamic Therapy. In Light-Responsive Nanostructured Systems for Applications in Nanomedicine; Springer: Cham, Switzerland, 2016; Volume 370, pp. 113–134. [Google Scholar] [CrossRef]
- Ding, D.; Guo, W.; Guo, C.; Sun, J.; Zheng, N.; Wang, F.; Yan, M.; Liu, S. MoO3−x quantum dots for photoacoustic imaging guided photothermal/photodynamic cancer treatment. Nanoscale 2017, 9, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective Cell Targeting with Light-Absorbing Microparticles and Nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, G.; Weissleder, R.; Hilderbrand, S.A. Upconverting Organic Dye Doped Core-Shell Nano-Composites for Dual-Modality NIR Imaging and Photo-Thermal Therapy. Theranostics 2013, 3, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wang, J.; Chen, C. Gold Nanorods Based Platforms for Light-Mediated Theranostics. Theranostics 2013, 3, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Kumar, S.; Li, G.; Zeng, C.; Kauffman, D.R.; Yoshimoto, J.; Iwasaki, Y.; Jin, R. Generation of Singlet Oxygen by Photoexcited Au25(SR)18 Clusters. Chem. Mater. 2014, 26, 2777–2788. [Google Scholar] [CrossRef]
- Hone, D.C.; Walker, P.I.; Evans-Gowing, R.; FitzGerald, S.; Beeby, A.; Chambrier, I.; Cook, M.J.; Russell, D.A. Generation of Cytotoxic Singlet Oxygen via Phthalocyanine-Stabilized Gold Nanoparticles: A Potential Delivery Vehicle for Photodynamic Therapy. Langmuir 2002, 18, 2985–2987. [Google Scholar] [CrossRef]
- Srivatsan, A.; Jenkins, S.V.; Jeon, M.; Wu, Z.; Kim, C.; Chen, J.; Pandey, R. Gold Nanocage-Photosensitizer Conjugates for Dual-Modal Image-Guided Enhanced Photodynamic Therapy. Theranostics 2014, 4, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennifer, M.; Connolly, V.R. Gold Nanosensitisers for Multimodal Optical Diagnostic Imaging and Therapy of Cancer. J. Nanomed. Nanotechnol. 2014, 5. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Tuchina, E.S.; Khanadeev, V.A.; Panfilova, E.V.; Petrov, P.O.; Tuchin, V.V.; Khlebtsov, N.G. Enhanced photoinactivation ofStaphylococcus aureuswith nanocomposites containing plasmonic particles and hematoporphyrin. J. Biophotonics 2013, 6, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.; Fu, H.; Wu, D.; Yang, Z. Nanofiller Reinforced Biodegradable PLA/PHA Composites: Current Status and Future Trends. Polymers 2018, 10, 505. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Bunghez, I.-R.; Iordache, S.M.; Badea, N.; Fierascu, R.-C.; Ion, R.M. Antioxidant Properties of Biohybrids Based on Liposomes and Sage Silver Nanoparticles. J. Nanosci. Nanotechnol. 2013, 13, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Kucková, L.; Jomová, K.; Švorcová, A.; Valko, M.; Segľa, P.; Moncoľ, J.; Kožíšek, J. Synthesis, Crystal Structure, Spectroscopic Properties and Potential Biological Activities of Salicylate‒Neocuproine Ternary Copper(II) Complexes. Molecules 2015, 20, 2115–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G.; et al. Plasmonic Copper Sulfide Nanocrystals Exhibiting Near-Infrared Photothermal and Photodynamic Therapeutic Effects. ACS Nano 2015, 9, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rashidi, L.H.; Yao, M.; Ma, L.; Chen, L.; Zhang, J.; Zhang, Y.; Chen, W. CuS nanoagents for photodynamic and photothermal therapies: Phenomena and possible mechanisms. Photodiagn. Photodyn. Ther. 2017, 19, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, X.; Li, H.; Zhao, Z.; Chi, X.; Huang, G.; Huang, D.; Liu, G.; Wang, X.; Gao, J. Theranostic Au Cubic Nano-aggregates as Potential Photoacoustic Contrast and Photothermal Therapeutic Agents. Theranostics 2014, 4, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Paschos, A.; Pandya, R.; Duivenvoorden, W.C.M.; Pinthus, J.H. Oxidative stress in prostate cancer: Changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 2013, 16, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Amirshaghaghi, A.; Huang, D.; Miller, J.; Stein, J.M.; Busch, T.M.; Cheng, Z.; Tsourkas, A. Protoporphyrin IX (PpIX)-Coated Superparamagnetic Iron Oxide Nanoparticle (SPION) Nanoclusters for Magnetic Resonance Imaging and Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1707030. [Google Scholar] [CrossRef] [PubMed]
- Tietze, R.; Lyer, S.; Dürr, S.; Alexiou, C. Nanoparticles for cancer therapy using magnetic forces. Nanomedicine 2012, 7, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.A.; Ménager, C.; Wilhelm, C. Combining Magnetic Hyperthermia and Photodynamic Therapy for Tumor Ablation with Photoresponsive Magnetic Liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Qi, T.; Liao, J.; Chu, B.; Yang, Q.; Qu, Y.; Li, W.; Li, H.; Luo, F.; Qian, Z. Mesoporous Magnetic Gold “Nanoclusters” as Theranostic Carrier for Chemo-Photothermal Co-therapy of Breast Cancer. Theranostics 2014, 4, 678–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal—Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal−organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Sun, Y.; Zhu, J.; Li, W.; Zhang, A.; Kuang, T.; Xie, J.; Yang, Z. Delivery of Nanoparticles for Treatment of Brain Tumor. Curr. Drug MeTable 2016, 17, 745–754. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Z.; Zhou, C.; Zhu, J.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yokel, R.A. The Chemical Species of Aluminum Influences Its Paracellular Flux across and Uptake into Caco-2 Cells, a Model of Gastrointestinal Absorption. Toxicol. Sci. 2005, 87, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warheit, D.B. Comparative Pulmonary Toxicity Assessment of Single-wall Carbon Nanotubes in Rats. Toxicol. Sci. 2003, 77, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.S.; Sharma, A. Nanoparticles aggravate heat stress induced cognitive deficits, blood–brain barrier disruption, edema formation and brain pathology. Prog. Brain Res. 2007, 162, 245–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xi, T.; Bai, J. Biological effects induced by nanosilver particles: In vivo study. Biomed. Mat. 2007, 2, S126–S128. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, T.-J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.-K.; Cho, M.H. Toxicity and Tissue Distribution of Magnetic Nanoparticles in Mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Taneda, S.; Taya, K.; Watanabe, G.; Li, X.; Fujitani, Y.; Nakajima, T.; Suzuki, A.K. Effects of in utero exposure to nanoparticle-rich diesel exhaust on testicular function in immature male rats. Toxicol. Lett. 2009, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Choi, Y.-J.; Song, H.; Kim, J.-H. Potential toxicity of engineered nanoparticles in mammalian germ cells and developing embryos: Treatment strategies and anticipated applications of nanoparticles in gene delivery. Hum. Reprod. Update 2016, 22, 588–619. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.J.; Zou, L.; Hartono, D.; Ong, C.N.; Bay, B.H.; Lanry Yung, L.Y. Gold Nanoparticles Induce Oxidative Damage in Lung Fibroblasts In Vitro. Adv. Mater. 2008, 20, 138–142. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-Eight-Day Oral Toxicity, Genotoxicity, and Gender-Related Tissue Distribution of Silver Nanoparticles in Sprague-Dawley Rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Jung, J.H.; Kim, S.S.; Yoon, J.-U.; Park, J.D.; Choi, B.S.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S.; et al. Twenty-Eight-Day Inhalation Toxicity Study of Silver Nanoparticles in Sprague-Dawley Rats. Inhal. Toxicol. 2008, 19, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles☆. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Connolly, M.; Fernández-Cruz, M.L.; Vijver, M.G.; Fernández, M.; Conde, E.; de Snoo, G.R.; Peijnenburg, W.J.G.M.; Navas, J.M. Species-specific toxicity of copper nanoparticles among mammalian and piscine cell lines. Nanotoxicology 2013, 8, 383–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to Copper Nanoparticles Causes Gill Injury and Acute Lethality in Zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lei, C.; Shen, Q.; Li, L.; Wang, M.; Guo, M.; Huang, Y.; Nie, Z.; Yao, S. Analysis of copper nanoparticles toxicity based on a stress-responsive bacterial biosensor array. Nanoscale 2013, 5, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Albrecht, R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001, 90, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S. Hyperthermia induced brain oedema: Current status & future perspectives. Indian J. Med. Res. 2006, 123, 629–652. [Google Scholar] [PubMed]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The Interaction of Manganese Nanoparticles with PC-12 Cells Induces Dopamine Depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, K.F.; Carrasco, A.; Powell, T.G.; Murr, L.E.; Garza, K.M. Biological effects of nanoparticulate materials. Mater. Sci. Eng. C 2006, 26, 1421–1427. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rahman, M.F.; Duhart, H.M.; Newport, G.D.; Patterson, T.A.; Murdock, R.C.; Hussain, S.M.; Schlager, J.J.; Ali, S.F. Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. NeuroToxicology 2009, 30, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Bleckmann, C.A.; Murdock, R.C.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Cellular Interaction of Different Forms of Aluminum Nanoparticles in Rat Alveolar Macrophages. J. Phys. Chem. B 2007, 111, 7353–7359. [Google Scholar] [CrossRef] [PubMed]

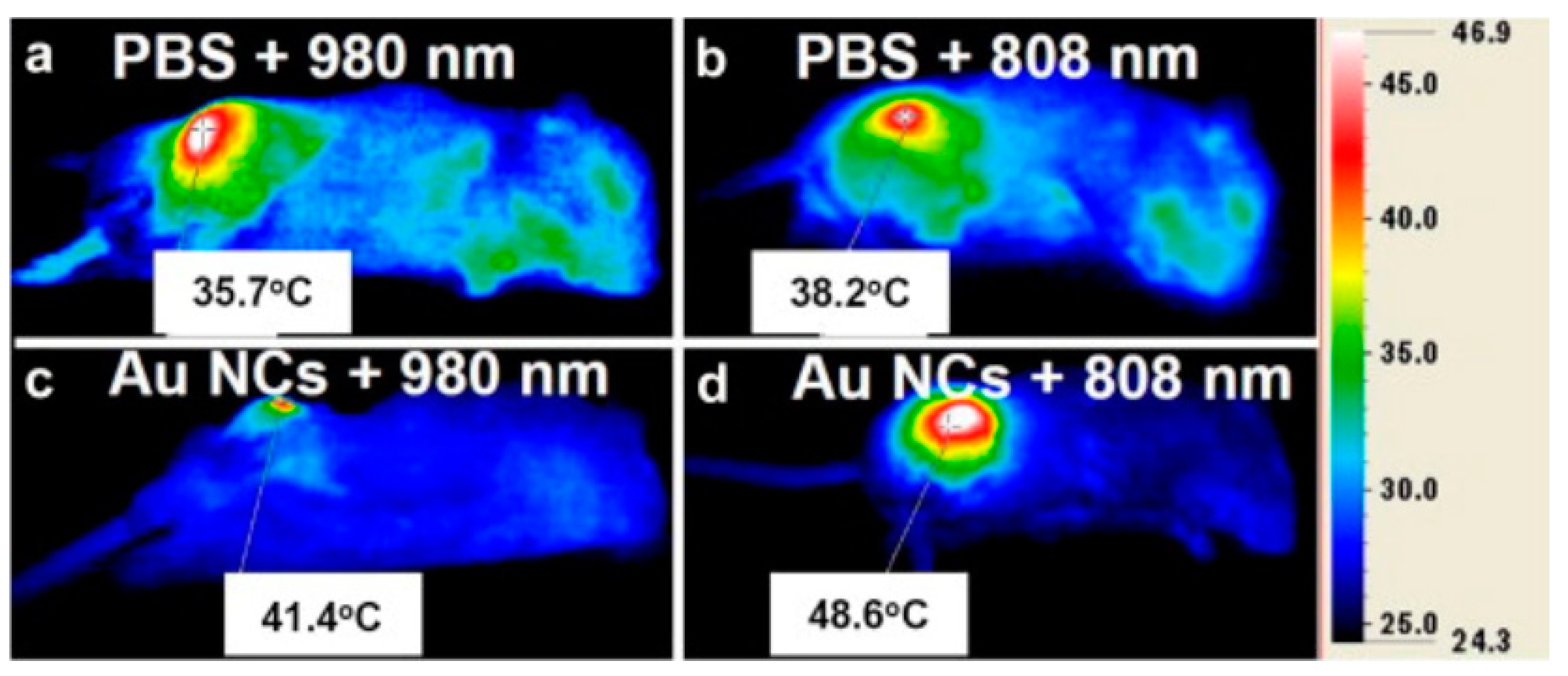
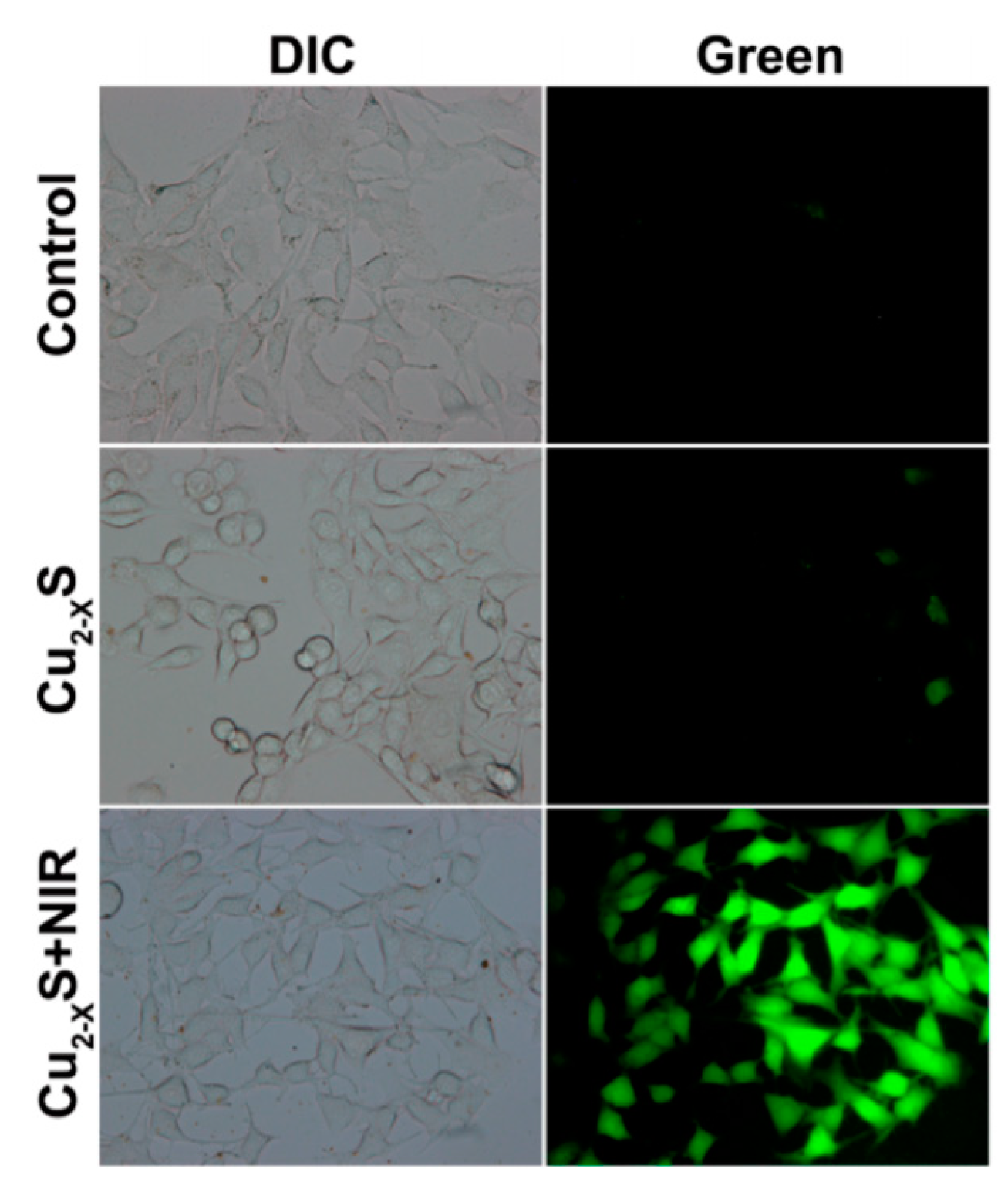
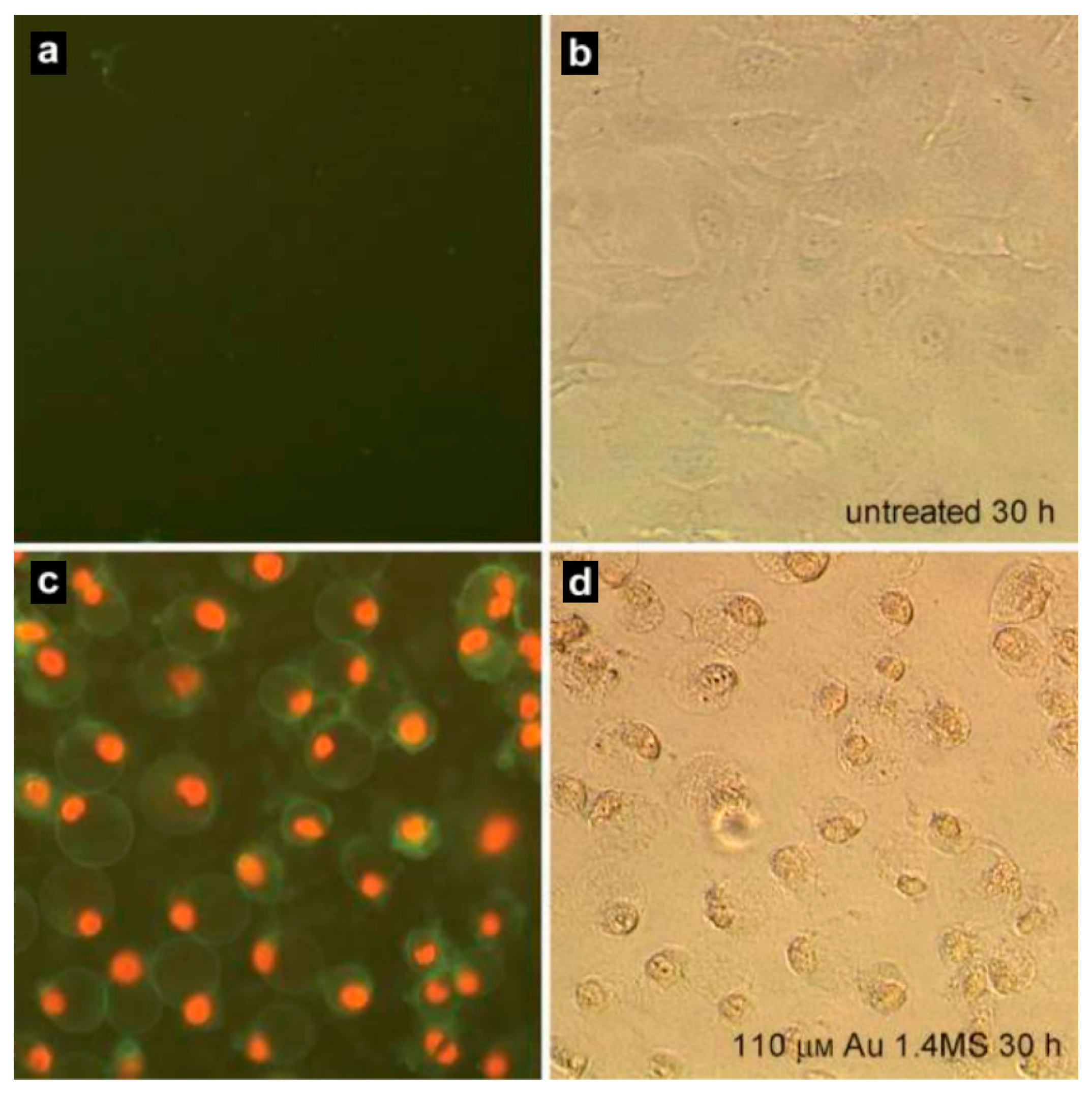
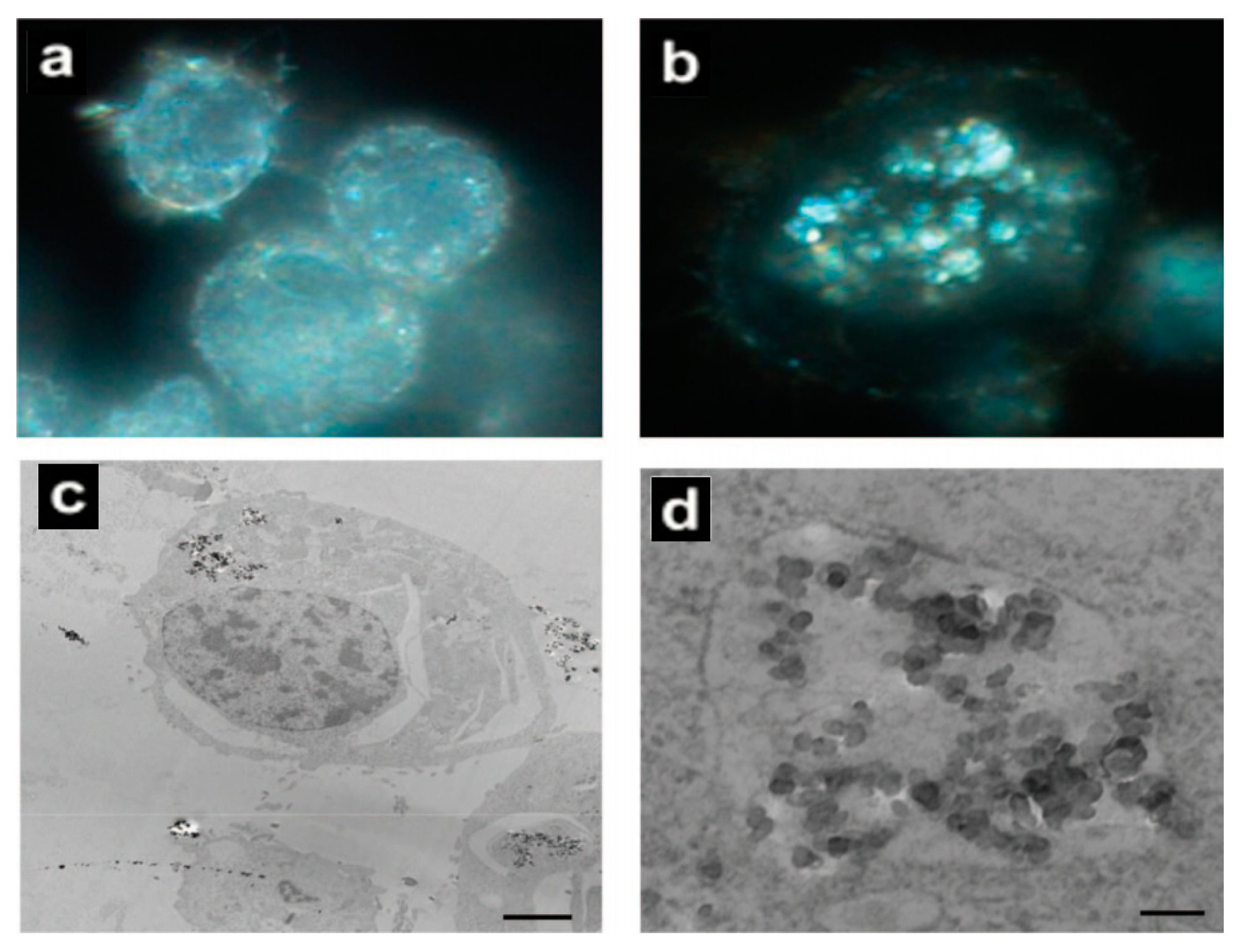
| Nanoparticles | Target | Dose | Result | References |
|---|---|---|---|---|
| Au | Mice (in vivo) | 2 × 105 PPB | Uptake of nanoparticles occurred in the small intestine | [157] |
| Ag | Zebrafish (in vivo) | 5–100 μg/mL | Dose-dependent toxicity in embryos | [158] |
| Ag, Cu, Al | Mice and Rat (in vivo) | 30–50 mg/kg | Blood-brain barrier penetration | [159] |
| Ag, Mn | PC-12 cells (in vitro) | 1–100 μg/mL | Cell shrinkage and irregular membrane | [160] |
| Ag, TiO2 | Murine macrophage cell line (in vitro) | 5 μg/mL | Aggregates of nanoparticles | [161] |
| TiO2 | Mice (in vivo) | 5 g/kg | Show histopathological effects | [162] |
| Cu, Mn | PC-12 cells (in vitro) | 10 μg/mL | Genes expression altered | [163] |
| Al, Al2O3 | Rat (in vivo) | 25–250 μg/mL | Phagocytosis hindered | [164] |
| Cu | Zebrafish (in vivo) | 0.25–1.5 mg/L | Biochemical, histopathological changes | [155] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy. Molecules 2018, 23, 1704. https://doi.org/10.3390/molecules23071704
Sun J, Kormakov S, Liu Y, Huang Y, Wu D, Yang Z. Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy. Molecules. 2018; 23(7):1704. https://doi.org/10.3390/molecules23071704
Chicago/Turabian StyleSun, Jingyao, Semen Kormakov, Ying Liu, Yao Huang, Daming Wu, and Zhaogang Yang. 2018. "Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy" Molecules 23, no. 7: 1704. https://doi.org/10.3390/molecules23071704





