Ultrasound-Assisted Extraction of Polysaccharides from Volvariella volvacea: Process Optimization and Structural Characterization
Abstract
:1. Introduction
2. Results and Discussion
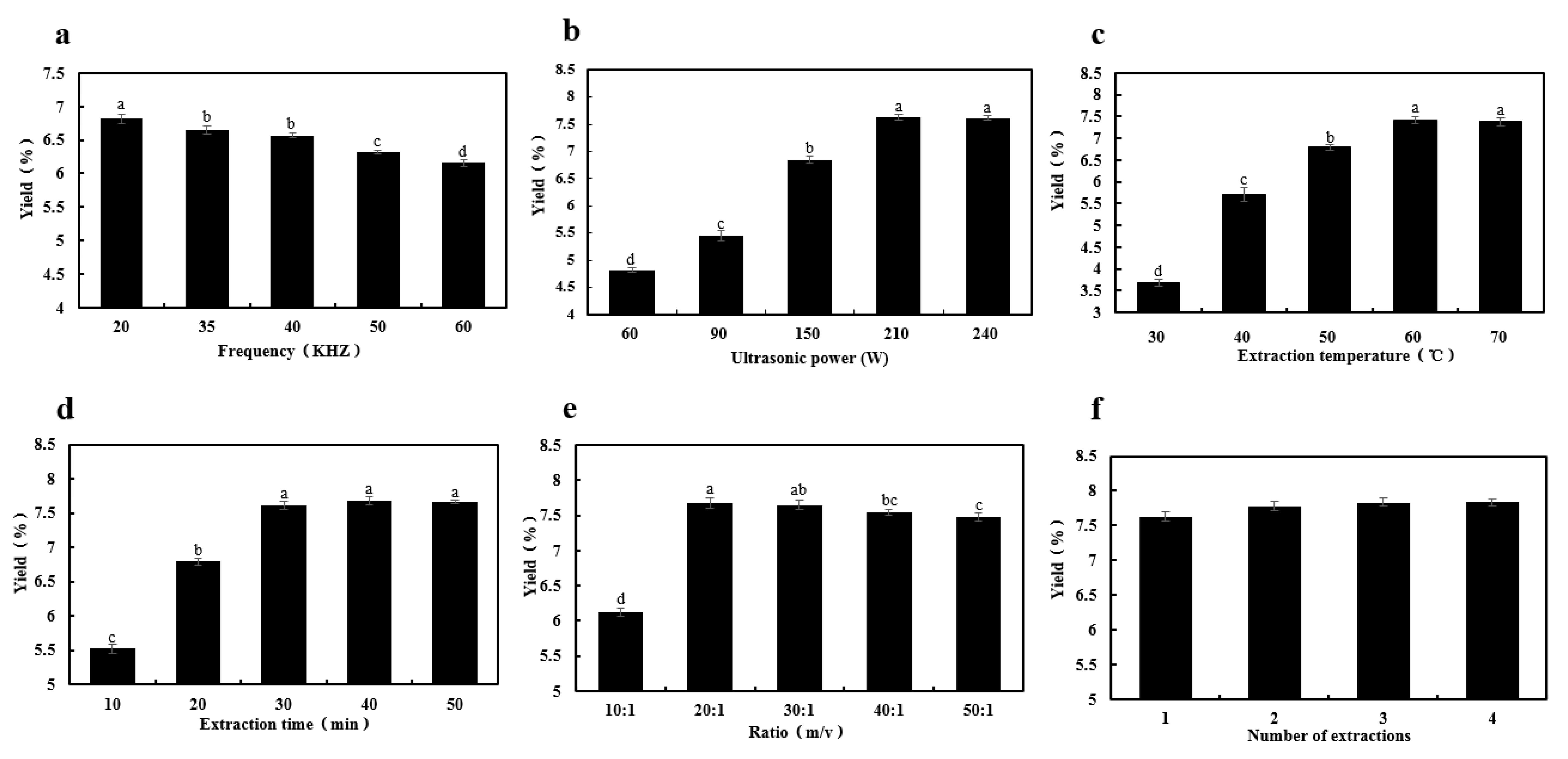
2.1. One-Factor-At-A-Time Experiments
2.1.1. Effect of Ultrasound Frequency
2.1.2. Effect of Ultrasound Power
2.1.3. Effect of Extraction Temperature
2.1.4. Effect of Extraction Time
2.1.5. Effect of Ratio of Water to Dried Fruiting Bodies
2.1.6. Effect of Number of Extractions
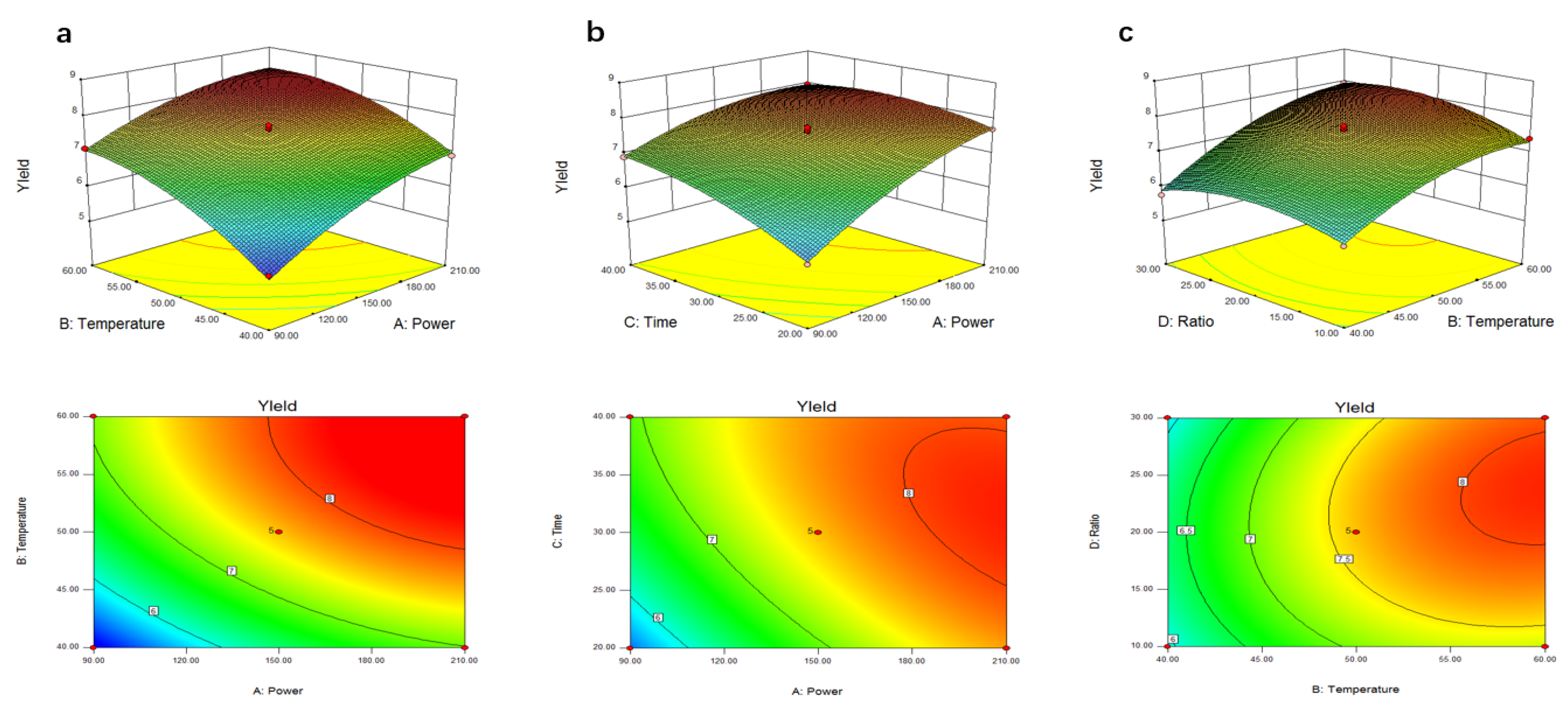
2.2. Optimization of the Parameters by RSM
Verification of Predictive Model
2.3. Comparison of the Structural Characteristics of Polysaccharides Extracted with Hot Water and Ultrasound-Assisted Methods
3. Materials and Methods
3.1. Materials
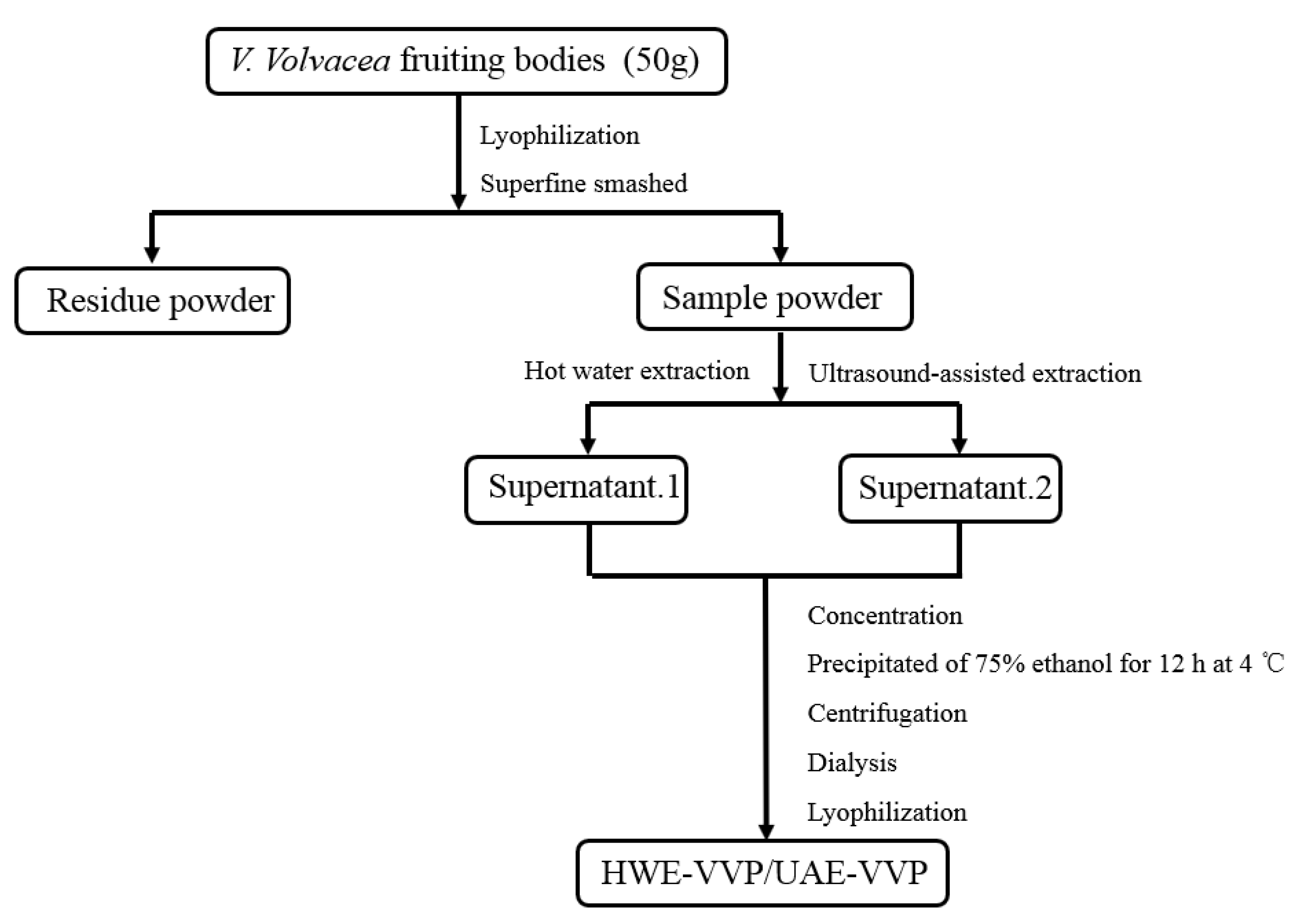
3.2. Polysaccharide Extraction
3.2.1. Hot Water Extraction (HWE)
3.2.2. Ultrasound-Assisted Extraction (UAE)
3.3. Optimization of Extraction Parameters
3.4. Analysis
3.4.1. Polysaccharide Yield
3.4.2. Carbohydrate Content and Monosaccharide Composition Analysis
3.4.3. Molecular Weight Analysis by Gel Permeation Chromatography
3.4.4. UV and FTIR
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 5–13. [Google Scholar]
- Zhang, B.B.; Hu, P.F.; Huang, J.; Hu, Y.D.; Chen, L.; Xu, G.R. Current advances on the structure, bioactivity, synthesis and metabolic regulation of novel ubiquinone derivatives in the edible and medicinal mushroom Antrodia cinnamomea. J. Agric. Food Chem. 2017, 65, 10395–10405. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Z.L.; Jia, A.R.; Shi, Y.P.; Li, R.H.; Yang, P.M. Extraction, preliminary characterization and evaluation of in vitro antitumor and antioxidant activities of polysaccharides from Menthe piperita. Int. J. Mol. Sci. 2014, 15, 16302–16319. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Yu, K.Q.; Liu, Y.Y.; Ouyang, M.Z.; Yan, M.H.; Luo, R.; Zhao, X.S. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae preparata. Int. J. Biol. Macromol. 2012, 50, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Lajili, S.; Deghrigue, M.; Bel, H.A.H.; Muller, C.D.; Bouraoui, A. In vitro immunomodulatory activity and in vivo anti-inflammatory and analgesic potential with gastroprotective effect of the Mediterranean red alga Laurencia obtusa. Pharm. Biol. 2016, 54, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Zhang, S.Q.; Ran, C.X.; Wang, L.S.; Kan, J.Q. Extraction, characterization and antioxidant activity of water-soluble polysaccharides from Tuber huidongense. Int. J. Biol. Macromol. 2016, 91, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Tang, W.; Jin, M.L.; Li, J.E.; Xie, M.Y. Recent advances in bioactive polysaccharides from Lycium barbarum L. Zizyphus jujuba Mill, Plantago spp. and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Cai, Y.J.; Chapman, S.J.; Buswell, J.A.; Chang, S.T. Production and distribution of endoglucanase, cellobiohydrolase, and β-glucosidase components of the cellulolytic system of Volvariella volvacea, the edible straw mushroom. Appl. Environ. Microbiol. 1999, 65, 553–559. [Google Scholar] [PubMed]
- Bao, D.; Gong, M.; Zheng, H.; Chen, M.; Zhang, L.; Wang, H.; Jiang, J.; Wu, L.; Zhu, Y.; Zhu, G.; et al. Sequencing and comparative analysis of the straw mushroom (Volvariella volvacea) genome. PLoS ONE 2013, 8, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Li, Y.; Chen, M.; Li, Z. Improved fruiting of the straw mushroom (Volvariella volvacea) on cotton waste supplemented with sodium acetate. Appl. Microbiol. Biotechnol. 2010, 101, 8533–8541. [Google Scholar] [CrossRef] [PubMed]
- Kishida, E.; Kinoshita, C.; Sane, Y.; Misaki, A. Structures and antitumor activities of polysaccharides isolated from mycelium of Volvariella volvacea. Biosci. Biotechnol. Biochem. 1992, 56, 1308–1309. [Google Scholar] [CrossRef] [PubMed]
- Misaki, A.; Motoko, N.; Yoshiaki, S.; Etsu, K.; Chigusa, K. Comparison of structure and antitumor activity of polysaccharides isolated from Fukurotake, the fruiting body of Volvariella volvacea. Agric. Biol. Chem. 1986, 50, 2171–2183. [Google Scholar] [CrossRef]
- Siu, K.C.; Xu, L.; Chen, X.; Wu, J.Y. Molecular properties and antioxidant activities of polysaccharides isolated from alkaline extract of wild Armillaria ostoyae mushrooms. Carbohydr. Polym. 2016, 137, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, G.M.; Kumar, S.S.; Giridhar, P.; Manohar, B. Antioxidant and cholesterol esterase inhibitory properties of supplementation with coconut water in submerged cultivation of the medicinal Chinese caterpillar mushroom, Ophiocordy cepssinensis cs 1197 (ascomycetes). Int. J. Med. Mushrooms 2017, 19, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, M.; Aldars-García, L.; Gil-Ramírez, A.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Pressurized water extraction of β-glucan enriched fractions with bile acids-binding capacities obtained from edible mushrooms. Biotechnol. Prog. 2014, 30, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Shahzor, G.K.; Liu, Y.; Saghir, A.S.; Wang, Y.F.; Aijaz, H.S.; Tian, X.; Homaida, M.A.; Huang, W. Optimization of enzyme assisted extraction of polysaccharides from Poriacocos. J. Med. Plants Res. 2017, 11, 331–337. [Google Scholar]
- Yang, N.; Jin, Y.; Jin, Z.; Xu, X. Electric-field-assisted extraction of garlic polysaccharides via experimental transformer device. Food Bioprocess Technol. 2016, 9, 1612–1622. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Morales, D.; Gil-Ramírez, A.; Jesus, L.I.D.; Gilbert-López, B.; Iacomini, M.; Soler-Rivas, C. Evaluation of microwave-assisted and pressurized liquid extractions to obtain β-d-glucans from mushrooms. Carbohydr. Polym. 2017, 156, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Aguiló-Aguayo, I.; Walton, J.; Viñas, I.; Tiwari, B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT-Food Sci. Technol. 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Zhang, Q.; Li, Y.F.; Dong, L.L.; Liu, S.L. Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohydr. Polym. 2015, 119, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Wang, W.D. Optimization of ultrasonic-assisted extraction and in vitro, antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Alzorqi, I.; Sudheer, S.; Lu, T.J.; Manickam, S. Ultrasonically extracted β-d-glucan from artificially cultivated mushroom, characteristic properties and antioxidant activity. Ultrason. Sonochem. 2017, 35, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, Y.Y.; Qiu, W.Y.; Wang, Z.B.; Ma, H.L. Ultrasound synergized with three-phase partitioning for extraction and separation of Corbicula fluminea polysaccharides and possible relevant mechanisms. Ultrason. Sonochem. 2017, 40, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.F.; Zhao, C.; Chen, X.; Chan, S.W.; Wu, J.Y. Chemical properties and bioactivities of goji (Lyceum barbarum) polysaccharides extracted by different methods. J. Funct. Foods 2015, 17, 903–909. [Google Scholar] [CrossRef]
- Zheng, Q.; Ren, D.; Yang, N.; Yang, X. Optimization for ultrasound-assisted extraction of polysaccharides with chemical composition and antioxidant activity from the Artemisia sphaerocephala Krasch seeds. Int. J. Biol. Macromol. 2016, 91, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Yang, Y.; Song, X.; Yu, Z. Optimization of ultrasound-assisted compound enzymatic extraction and characterization of polysaccharides from blackcurrant. Carbohydr. Polym. 2015, 117, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Afshari, K.; Samavati, V.; Shahidi, S.A. Ultrasonic-assisted extraction and in-vitro antioxidant activity of polysaccharide from Hibiscus leaf. Int. J. Biol. Macromol. 2015, 74, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Chen, Y.; Du, X.J.; Zhang, Y.; Liu, X.H.; Wang, X.D. Ultrasound extraction optimization, structural features, and antioxidant activity of polysaccharides from Tricholoma matsutake. J. Zhejiang Univ. Sci. B 2017, 18, 674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Zhao, B.; Wang, X.; Wu, Y.; Yao, J. A comparison study on microwave-assisted extraction of Potentilla anserina L. polysaccharides with conventional method: Molecule weight and antioxidant activities evaluation. Carbohydr. Polym. 2010, 80, 84–93. [Google Scholar] [CrossRef]
- Yan, J.K.; Pei, J.J.; Ma, H.L.; Wang, Z.B. Effects of ultrasound on molecular properties, structure, chain conformation and degradation kinetics of carboxylic curdlan. Carbohydr. Polym. 2015, 121, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.M.; Luo, Z.S.; Tao, B.P.; Chen, C. Ultrasonic-assisted extraction and purification of phenolic compounds from sugarcane (Saccharum officinarum L.) rind. LWT-Food Sci. Technol. 2015, 60, 970–976. [Google Scholar] [CrossRef]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohydr. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, W.P.; Zhang, H.S.; Huang, Q. Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from boletus edulis, mycelia using response surface methodology. Carbohydr. Polym. 2012, 87, 614–619. [Google Scholar] [CrossRef]
- Dean, A.; Voss, D.; Draguljić, D. Design and Analysis of Experiments; Springer: New York, NY, USA, 1999. [Google Scholar]
- Cui, F.J.; Li, Y.; Xu, Z.H.; Xu, H.Y.; Sun, K.; Tao, W.Y. Optimization of the medium composition for production of mycelial biomass and exo-polymer by Grifola frondosa GF9801 using response surface methodology. Bioresour. Technol. 2006, 97, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Villetti, M.A.; Crespo, J.S.; Soldi, M.S.; Pires, A.T.N.; Borsali, R.; Soldi, V. Thermal degradation of natural polymers. J. Therm. Anal. Calorim. 2002, 67, 295–303. [Google Scholar] [CrossRef]
- Yasuo, I.; Toru, T.; Kyuichi, Y.; Atsuya, T.; Teruyuki, K. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization. Innov. Food Sci. Emerg. 2008, 9, 140–146. [Google Scholar]
- Cheung, Y.C.; Siu, K.C.; Liu, Y.S.; Wu, J.Y. Molecular properties and antioxidant activities of polysaccharide–protein complexes from selected mushrooms by ultrasound-assisted extraction. Process Biochem. 2012, 47, 892–895. [Google Scholar] [CrossRef]
- Colussi, R.; Pinto, V.Z.; Halal, S.L.M.E.; Vanier, N.L.; Villanova, F.A.; Silva, R.M.E.; da Rosa Zavareze, E.; Dias, A.R. Structural, morphological, and physicochemical properties of acetylated high-, medium-, and low-amylose rice starches. Carbohydr. Polym. 2014, 103, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Characteristics and properties of hydroxypropylated rice starch based biodegradable films. Food Hydrocol. 2015, 50, 54–64. [Google Scholar] [CrossRef]
- Sudharsan, K.; Chandra, M.C.; Azhagu, S.B.P.; Archana, G.; Sabina, K.; Sivarajan, M.; Sukumar, M. Production and characterization of cellulose reinforced starch (crt) films. Int. J. Biol. Macromol. 2016, 83, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, G.; Yu, Z.; Song, X.; Li, X.; Yang, Y.; Wang, L.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2016, 199, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, F.M.; Cui, F.J.; Sun, W.J.; Zhang, J.S.; Qian, L.S.; Yang, Y.; Wu, D.; Dong, Y.; Jiang, J.; et al. Improved postharvest quality and respiratory activity of straw mushroom (Volvariella volvacea) with ultrasound treatment and controlled relative humidity. Sci. Hortic. 2017, 225, 56–64. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008.
- Barrientos, R.C.; Clerigo, M.M.; Paano, A.M.C. Extraction, isolation and MALDI-QTOFMS/MS analysis of β-d-glucan from the fruiting bodies of Daedalea quercina. Int. J. Biol. Macromol. 2016, 93, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Wu, L.; Zheng, L.; Wang, W.Z.; Zhang, X.J.; Jin, L.Q.; Zheng, Y.G. Biosynthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate by carbonyl reductase from Rhodosporidium toruloides in mono and biphasic media. Bioresour. Technol. 2018, 249, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Dong, S.C.; Yin, H.H.; Xue, Y.P.; Tang, X.L.; Zhang, X.J.; He, J.Y.; Zheng, Y.G. Enzymatic synthesis of an ezetimibe intermediate using carbonyl reductase coupled with glucose dehydrogenase in an aqueous-organic solvent system. Bioresour. Technol. 2017, 229, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Lu, M.M.; Zhang, X.H.; Cheng, F.; Xu, J.M.; Xue, Y.P.; Jin, L.Q.; Wang, Y.S.; Zheng, Y.G. Significant improvement of the nitrilase activity by semi-rational protein engineering and its application in the production of iminodiacetic acid. Int. J. Biol. Macromol. 2018, 116, 563–571. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Run | Ultrasound Power (W, X1) | Extraction Temperature (Degrees C, X2) | Extraction Time (min, X3) | Extraction Ratio (mL/g, X4) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 90(−1) | 50(0) | 30(0) | 30(1) | 6.08 ± 0.23 |
| 2 | 90(−1) | 60(1) | 30(0) | 20(0) | 7.10 ± 0.31 |
| 3 | 210(1) | 50(0) | 40(1) | 20(0) | 7.95 ± 0.18 |
| 4 | 150(0) | 50(0) | 30(0) | 20(0) | 7.54 ± 0.30 |
| 5 | 150(0) | 40(−1) | 40(1) | 20(0) | 6.55 ± 0.28 |
| 6 | 90(−1) | 50(0) | 20(−1) | 20(0) | 5.42 ± 0.36 |
| 7 | 150(0) | 50(0) | 30(0) | 20(0) | 7.51 ± 0.30 |
| 8 | 150(0) | 50(0) | 30(0) | 20(0) | 7.65 ± 0.24 |
| 9 | 150(0) | 50(0) | 20(−1) | 30(1) | 6.64 ± 0.28 |
| 10 | 90(−1) | 50(0) | 40(1) | 20(0) | 6.90 ± 0.23 |
| 11 | 90(−1) | 50(0) | 30(0) | 10(−1) | 5.97 ± 0.33 |
| 12 | 210(1) | 60(1) | 30(0) | 20(0) | 8.21 ± 0.35 |
| 13 | 210(1) | 40(−1) | 30(0) | 20(0) | 6.89 ± 0.38 |
| 14 | 150(0) | 50(0) | 30(0) | 20(0) | 7.75 ± 0.27 |
| 15 | 210(1) | 50(0) | 30(0) | 30(1) | 7.96 ± 0.19 |
| 16 | 150(0) | 40(−1) | 20(−1) | 20(0) | 5.74 ± 0.27 |
| 17 | 150(0) | 60(1) | 30(0) | 30(1) | 7.94 ± 0.33 |
| 18 | 150(0) | 40(−1) | 30(0) | 10(−1) | 5.88 ± 0.21 |
| 19 | 150(0) | 50(0) | 40(1) | 10(−1) | 7.08 ± 0.26 |
| 20 | 150(0) | 50(0) | 40(1) | 30(1) | 7.68 ± 0.31 |
| 21 | 150(0) | 50(0) | 30(0) | 20(0) | 7.68 ± 0.24 |
| 22 | 150(0) | 60(1) | 40(1) | 20(0) | 8.07 ± 0.28 |
| 23 | 150(0) | 60(1) | 30(0) | 10(−1) | 7.38 ± 0.37 |
| 24 | 150(0) | 40(−1) | 30(0) | 30(1) | 5.75 ± 0.21 |
| 25 | 210(1) | 50(0) | 30(0) | 10(−1) | 7.70 ± 0.24 |
| 26 | 210(1) | 50(0) | 20(−1) | 20(0) | 7.69 ± 0.32 |
| 27 | 90(−1) | 40(−1) | 30(0) | 20(0) | 5.13 ± 0.27 |
| 28 | 150(0) | 60(1) | 20(−1) | 20(0) | 7.48 ± 0.19 |
| 29 | 150(0) | 50(0) | 20(−1) | 10(−1) | 6.22 ± 0.24 |
| Molar Ratio | |||
|---|---|---|---|
| d-Mannose (22.008 min) | d-Glucose (22.898 min) | d-Galactose (23.545 min) | |
| UAE-VVP | 1 | 6.58 | 1.12 |
| HWE-VVP | 1 | 4.79 | 1.14 |
| Samples | Peak No | Retention Time (min) | MW (KDa) | Area Percentages (%) |
|---|---|---|---|---|
| HWE-VVP | 1 | 13.41 | 357.56 | 92.08 |
| 2 | 16.98 | 2.47 | 7.92 | |
| UAE-VVP | 1 | 13.17 | 255.42 | 24.55 |
| 2 | 16.95 | 2.69 | 46.87 | |
| 3 | 19.37 | 0.12 | 28.58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, F.-J.; Qian, L.-S.; Sun, W.-J.; Zhang, J.-S.; Yang, Y.; Li, N.; Zhuang, H.-N.; Wu, D. Ultrasound-Assisted Extraction of Polysaccharides from Volvariella volvacea: Process Optimization and Structural Characterization. Molecules 2018, 23, 1706. https://doi.org/10.3390/molecules23071706
Cui F-J, Qian L-S, Sun W-J, Zhang J-S, Yang Y, Li N, Zhuang H-N, Wu D. Ultrasound-Assisted Extraction of Polysaccharides from Volvariella volvacea: Process Optimization and Structural Characterization. Molecules. 2018; 23(7):1706. https://doi.org/10.3390/molecules23071706
Chicago/Turabian StyleCui, Feng-Jie, Li-Sun Qian, Wen-Jing Sun, Jin-Song Zhang, Yan Yang, Na Li, Hai-Ning Zhuang, and Di Wu. 2018. "Ultrasound-Assisted Extraction of Polysaccharides from Volvariella volvacea: Process Optimization and Structural Characterization" Molecules 23, no. 7: 1706. https://doi.org/10.3390/molecules23071706




