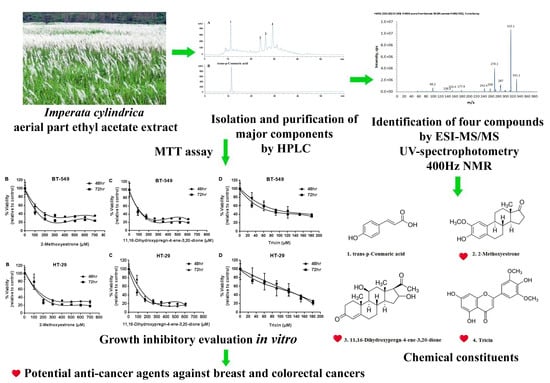
Identification and Growth Inhibitory Activity of the Chemical Constituents from Imperata Cylindrica Aerial Part Ethyl Acetate Extract
Abstract
:1. Introduction
2. Results
2.1. Isolation, Identification, and Quantification of Compounds (1)–(4)
2.1.1. Compound (1): Trans-p-Coumaric Acid
2.1.2. Compound (2): 2-Methoxyestrone
2.1.3. Compound (3): 11, 16-Dihydroxypregn-4-ene-3, 20-dione
2.1.4. Compound (4): Tricin
2.1.5. Content of Analytes in IMP Aerial Part Ethyl Acetate Extract
2.2. Growth Inhibitory Evaluation of Compounds (1)–(4) on Breast Cancer and Colorectal Cancer In Vitro
3. Discussion
4. Materials and Methods
4.1. Cells, Chemicals and Reagents
4.2. Preparation of Powder Extract of IMP Aerial Part
4.3. HPLC Analysis
4.4. Isolation and Purification of Compounds (1)–(4) by HPLC
4.5. Mass Spectrometry
4.6. Ultraviolet-Visible Spectrophotometry
4.7. NMR Analysis
4.8. Quantitative Analysis
4.9. MTT Assay
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pinilla, V.; Luu, B. Isolation and partial characterization of immunostimulating polysaccharides from Imperata cylindrica. Planta Med. 1999, 65, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Eussen, J.H.H.; Niemann, G.J. Growth inhibiting substances from leaves of Imperata cylindrica (L.) Beauv. Z. Pflanzenphysiol. 1981, 102, 263–266. [Google Scholar] [CrossRef]
- Matsunaga, K.; Shibuya, M.; Ohizumi, Y. Graminone B, a novel lignan with vasodilative activity from Imperata cylindrica. J. Nat. Prod. 1994, 57, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Shibuya, M.; Ohizumi, Y. Imperanene, a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica. J. Nat. Prod. 1995, 58, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Abdel-Lateff, A.; Fouad, M.A.; Ibrahim, S.R.; Elkhayat, E.S.; Okino, T. Chemical composition and hepato-protective activity of Imperata cylindrica Beauv. Pharmacogn. Mag. 2009, 5, 28–36. [Google Scholar]
- Liu, R.H.; Chen, S.S.; Ren, G.; Shao, F.; Huang, H.L. Phenolic compounds from roots of Imperata cylindrica var. major. Chin. Herb. Med. 2013, 5, 240–243. [Google Scholar] [CrossRef]
- An, H.J.; Nugroho, A.; Song, B.M.; Park, H.J. Isoeugenin, a novel nitric oxide synthase inhibitor isolated from the rhizomes of Imperata cylindrica. Molecules 2015, 20, 21336–21345. [Google Scholar] [CrossRef] [PubMed]
- Tine, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. A Method for LC-MS/MS profiling of coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler extracts and essential oils. Molecules 2017, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Pramanik, B.N.; Liu, Y.H.; Mirza, U.A. Applications of LC/MS in structure identifications of small molecules and proteins in drug discovery. J. Mass Spectrom. 2007, 42, 279–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, A.H.Y.; Wang, Y.; Ho, W.S. Cytotoxic and pro-oxidative effects of Imperata cylindrica aerial part ethyl acetate extract in colorectal cancer in vitro. Phytomedicine 2016, 23, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Zhu, F.Y.; Chan, W.L.; Liu, H.; Lo, C. Cytochrome P450 93G1 is a flavone synthase II that channels flavanones to the biosynthesis of Tricin O-linked conjugates in rice. Plant Physiol. 2014, 165, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, Y.; Yoo, C.G.; Ragauskas, A.J. The occurrence of Tricin and its derivatives in plants. Green Chem. 2016, 18, 1439–1454. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kim, C.M. Antioxidant constituents from the stem of Sorghum bicolor. Arch. Pharm. Res. 2003, 26, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Xu, X.; Zhou, B.; Hu, F.; Zhang, C.; Zhang, M. Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens. Phytochemistry 2004, 65, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Guerriero, E.; Volpe, M.G.; Capone, F.; La Cara, F.; Ciliberto, G.; Colonna, G.; Costantini, S. Differential response of two human breast cancer cell lines to the phenolic extract from flaxseed oil. Molecules 2016, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic acids are absorbed from the rat stomach with different absorption rates. J. Agric. Food Chem. 2006, 54, 7539–7543. [Google Scholar] [CrossRef] [PubMed]
- Dobos, J.; Tímár, J.; Bocsi, J.; Burián, Z.; Nagy, K.; Barna, G.; Peták, I.; Ladányi, A. In vitro and in vivo anti-tumor effect of 2-methoxyestradiol on human melanoma. Int. J. Cancer 2004, 112, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.J.; Sparreboom, A.; Xu, X. Characterization of in vitro and in vivo metabolic pathways of the investigational anticancer agent, 2-methoxyestradiol. J. Pharm. Sci. 2007, 96, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.T.; Conney, A.H. Is 2-Methoxyestradiol an endogenous estrogen metabolite that inhibitsmammary carcinogenesis? Cancer. Res. 1998, 58, 2269–2277. [Google Scholar] [PubMed]
- Choudhary, M.I.; Nasir, M.; Khan, S.N.; Atif, M.; Ali, R.A.; Khalil, S.M.; Rahman, A. Microbial hydroxylation of hydroxyprogesterones and α-glucosidase inhibition activity of their metabolites. Z. Naturforsch. 2007, 62, 593–599. [Google Scholar] [CrossRef]
- Lee, D.E.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomic profiles of Aspergillus oryzae and Bacillus amyloliquefaciens during rice koji fermentation. Molecules 2016, 21, 773. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.J.; Zhang, Y.; Liu, C.M.; Liu, J.E.; Wu, X.Q.; Zhang, Y. Separation and purification of Tricin from an antioxidant product derived from bamboo leaves. J. Agric. Food Chem. 2007, 55, 10086–10092. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomarkers. Prev. 2000, 9, 1163–1170. [Google Scholar] [PubMed]
- Cai, H.; Hudson, E.A.; Mann, P.; Verschoyle, R.D.; Greaves, P.; Manson, M.M.; Steward, W.P.; Gescher, A.J. Growth-inhibitory and cell cycle-arresting properties of the rice bran constituent Tricin in human-derived breast cancer cells in vitro and in nude mice in vivo. Br. J. Cancer 2004, 91, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Verschoyle, R.D.; Greaves, P.; Cai, H.; Borkhardt, A.; Broggini, M.; D’Incalci, M.; Riccio, E.; Doppalapudi, R.; Kapetanovic, I.M.; Steward, W.P.; et al. Preliminary safety evaluation of the putative cancer chemopreventive agent Tricin, a naturally occurring flavone. Cancer Chemother. Pharmacol. 2006, 57, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Ibrahim, R.K. Tricin-a potential multifunctional nutraceutical. Phytochem. Rev. 2010, 9, 413–424. [Google Scholar] [CrossRef]
- Jang, M.H.; Ho, J.K.; Hye, J.J. Tricin, 4′,5′,7′-trihydroxy-3′,5′-dimethoxyflavone, exhibits potent antiangiogenic activity in vitro. Int. J. Oncol. 2016, 49, 1497–1504. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Shen, J.Z.; Wang, Y.; Lu, A.X.; Ho, W.S. Anti-oxidant and anti-cancer activities of Angelica dahurica extract via induction of apoptosis in colon cancer cells. Phytomedicine 2016, 15, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |










| Analyte | Ion Mode | Molecular Formula | CAS No. | MS/MS Fragments (m/z) |
|---|---|---|---|---|
| trans-p-Coumaric acid | [M+H]+ | C9H8O3 | 501-98-4 | 165.7, 147.4, 136.3, 118.9, 90.5 |
| 2-Methoxyestrone | [M-H]− | C19H24O3 | 362-08-3 | 299.9, 285.0, 271.8, 256.3, 243.8 |
| 11, 16-Dihydroxypregn-4-ene-3, 20-dione | [M-H]− | C21H30O4 | 55622-61-2 | 344.9, 327.0, 309.1, 290.9, 265.2, 247.0 |
| Tricin | [M+H]+ | C17H14O7 | 520-32-1 | 331.1, 315.0, 287.0, 270.1, 258.0, 242.4 |
| Analytes | Calibration Curves a | R2 b | Linear Range (mg/mL) | LOD c (µg/mL) | LOQ d (µg/mL) | Contents of Analytes (mg/g Extract, n = 3) |
|---|---|---|---|---|---|---|
| trans-p-Coumaric acid | y = 114751x − 31.91 | 0.9999 | 0.0010–0.25 | 0.30 | 0.95 | 0.12 ± 0.010 |
| 2-Methoxyestrone | y = 13217x + 195.65 | 0.9992 | 0.031–1.00 | 7.28 | 24.84 | 0.86 ± 0.042 |
| 11,16-Dihydroxypregn-4-ene-3, 20-dione | y = 15063x + 1173.70 | 0.9995 | 0.12–2.50 | 6.71 | 22.91 | 0.65 ± 0.13 |
| Tricin | y = 35025x − 126.29 | 0.9999 | 0.016–2.00 | 3.23 | 11.02 | 0.59 ± 0.041 |
| Cancer Unit | Treatment Group (48/72 h) | ||||
|---|---|---|---|---|---|
| Trans-p-Coumaric Acid | 2-Methoxyestrone | 11,16-Dihydroxypregn-4-ene-3, 20-dione | Tricin | ||
| BT-549 | µg/mL | 151/83 | 43/31 | 35/34 | 31/23 |
| µM | 920/507 | 144/102 a | 101/97 a | 95/68 a | |
| HT-29 | µg/mL | 135/135 | 51/44 | 33/46 | 39/38 |
| µM | 821/821 | 169/147 a | 96/134 a | 118/114 a | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shen, J.Z.; Chan, Y.W.; Ho, W.S. Identification and Growth Inhibitory Activity of the Chemical Constituents from Imperata Cylindrica Aerial Part Ethyl Acetate Extract. Molecules 2018, 23, 1807. https://doi.org/10.3390/molecules23071807
Wang Y, Shen JZ, Chan YW, Ho WS. Identification and Growth Inhibitory Activity of the Chemical Constituents from Imperata Cylindrica Aerial Part Ethyl Acetate Extract. Molecules. 2018; 23(7):1807. https://doi.org/10.3390/molecules23071807
Chicago/Turabian StyleWang, Yan, James Zheng Shen, Yuk Wah Chan, and Wing Shing Ho. 2018. "Identification and Growth Inhibitory Activity of the Chemical Constituents from Imperata Cylindrica Aerial Part Ethyl Acetate Extract" Molecules 23, no. 7: 1807. https://doi.org/10.3390/molecules23071807



_Shen.jpg)