Correlation Analysis of Phenolic Contents and Antioxidation in Yellow- and Black-Seeded Brassica napus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of Total Phenolic and Flavonoid Content in Developing Seeds of Yellow- and Black-Seeded B. napus
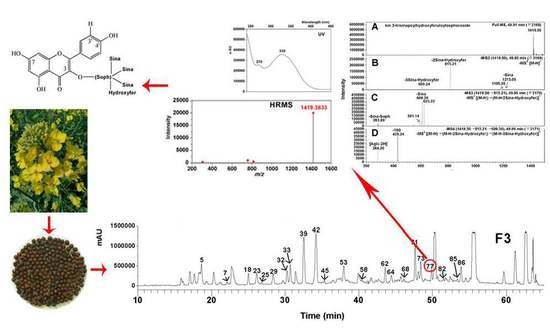
2.2. HPLC–DAD–ESI/MS Analysis of Phenolic Compounds in Yellow- and Black-Seeded B. napus
2.3. Antioxidant Activity of Yellow- and Black-Seeded B. napus
2.4. Correlation Analysis of Phenolic Compounds and Antioxidant Activity
3. Experimental Section
3.1. Plant Materials
3.2. Extraction of Seed Polyphenols
3.3. Quantification of Soluble Phenolic Acids and Flavonoids
3.4. HPLC–DAD–ESI/MS Analysis of Polyphenolic Components
3.5. Antioxidant Activity Analysis
3.6. Statistics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolfram, K.; Schmidt, J.; Wray, V.; Milkowski, C.; Schliemann, W.; Strack, D. Profiling of phenylpropanoids in transgenic low-sinapine oilseed rapeseed (Brassica napus). Phytochemistry 2010, 71, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, L.; Pu, H.; Li, C.; Hu, Q. Profiles and distribution of soluble and insoluble phenolics in Chinese rapeseed (Brassica napus). Food Chem. 2012, 135, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Reichelt, M.; Kai, M.; Schneider, B. Metabolic profiling of lignins and other secondary metabolites from rapeseed (Brassica napus L.). J. Agric. Food Chem. 2012, 60, 10523–10529. [Google Scholar] [CrossRef] [PubMed]
- Tayo, T.; Dutta, N.; Sharma, K. Effect of feeding canola quality rapeseed mustard meal on animal production—A review. Agric. Rev. 2012, 33, 114–121. [Google Scholar]
- Nour-Eldin, H.H.; Madsen, S.R.; Engelen, S.; Jorgensen, M.E.; Olsen, C.E.; Andersen, J.S.; Seynnaeve, D.; Verhoye, T.; Fulawka, R.; Denolf, P.; et al. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters. Nat. Biotechnol. 2016, 35, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y. Molecular mechanism of manipulating seed coat coloration in oilseed Brassica species. J. Appl. Genet. 2013, 54, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Lipsa, F.D.; Snowdon, R.; Friedt, W. Quantitative genetic analysis of condensed tannins in oilseed rape meal. Euphytica 2012, 184, 195–205. [Google Scholar] [CrossRef]
- Jiang, J.J.; Shao, Y.L.; Li, A.M.; Lu, C.L.; Zhang, Y.T.; Wang, Y.P. Flavonoid composition analysis and gene expression in developing seeds of yellow- and black-seeded Brassica napus. J. Integr. Plant Biol. 2013, 55, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Wang, Y.; Xie, T.; Rong, H.; Li, A.M.; Fang, Y.J.; Wang, Y.P. Metabolic characteristics in meal of black rapeseed and yellow-seeded progeny of Brassica napus-Sinapis alba hybrids. Molecules 2015, 20, 21204–21213. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Stein, A.; Wittkop, B.; Sarvari, P.; Li, J.; Yan, X.; Dreyer, F.; Frauen, M.; Friedt, W.; Snowdon, R.J. A knockout mutation in the lignin biosynthesis gene CCR1 ezplains a major QTL for acid detergent lignin content in Brassica napus seeds. Theor. Appl. Genet. 2012, 124, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhu, L.X.; Qi, L.P.; Ke, H.M.; Yi, B.; Shen, J.X.; Tu, J.X.; Ma, C.Z.; Fu, T.D. Characterization of interploid hybrids from crosses between Brassica juncea and B. oleracea and the production of yellow-seeded B. napus. Theor. Appl. Genet. 2012, 125, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Jiang, J.; Zhang, Y.; Snowdon, R.J.; Liang, G.; Wang, Y. Molecular and cytological characterization of introgression lines in yellow seed derived from somatic hybrids between Brassica napus and Sinapis alba. Mol. Breed. 2012, 29, 209–219. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Hu, Z.; Zhu, M.; Zhu, Z.; Wang, Z.; Tian, S.; Chen, G. Anthocyanin accumulation and molecular analysis of correlated genes in purple kohlrabi (Brassica oleracea var. gongylodes L.). J. Agric. Food Chem. 2015, 63, 4160–4169. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Pajak, P.; Socha, R.; Galkowska, D.; Roznowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; Bohr, V.A.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural polyphenols as sirtuin 6 modulators. Sci. Rep. 2018, 8, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- Agudo, A.; Cabrera, L.; Amiano, P.; Ardanaz, E.; Barricarte, A.; Berenguer, T.; Chirlaque, M.D.; Dorronsoro, M.; Jakszyn, P.; Larranaga, N.; et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in spanish adults: Findings from the spanish cohort of the european prospective investigation into cancer and nutrition (EPIC-Spain). Am. J. Clin. Nutr. 2007, 85, 1634−1642. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharm. Res. 2007, 55, 224−236. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Gallicchio, L.; Lindsley, K.; Shiels, M.; Hammond, E.; Tao, X.G.; Chen, L.; Robinson, K.A.; Caulfield, L.E.; Herman, J.G.; et al. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol. Biomark. Prev. 2009, 18, 184−195. [Google Scholar] [CrossRef] [PubMed]
- Rugina, D.; Sconta, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extractes and the cytotoxic action of their anthocyanin fraction in HeLa human cervical tumor cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hua, W.; Yang, H.; Guo, T.; Sun, X.; Wang, X.; Liu, G.; Wang, H. Effects of specific organs on seed oil accumulation in Brassica napus L. Plant Sci. 2014, 227, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Augar, B.; Marnet, N.; Gautier, V.; Maia-Grondard, A.; Leprince, F.; Renard, M.; Guyot, S.; Nesi, N.; Routaboul, J.M. A detailed survey of seed coat flavonoids in developing seeds of Brassica napus L. J. Agric. Food Chem. 2010, 58, 6246–6256. [Google Scholar] [CrossRef] [PubMed]
- Badani, A.G.; Snowdon, R.J.; Wittkop, B.; Lipsa, F.D.; Baetzel, R.; Horn, R.; De Haro, A.; Font, R.; Luhs, W.; Friedt, W. Colocalization of a partially dominant gene for yellow seed colour with a major QTL influencing acid detergent fibre (ADF) content in different crosses of oilseed rape (Brassica napus). Genome 2006, 49, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Fu, F.; Lu, K.; Zhang, K.; Wang, R.; Xu, X.; Wang, M.; Lu, J.; Wan, H.; Tang, Z.; et al. Differential accumulation of phenolic compounds and expression of related genes in black- and yellow-seeded Brassica napus. J. Exp. Bot. 2013, 64, 2885–2898. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Chapple, C. The phenylproponoid pathway in Arabidopsis. Arabidopsis Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed]
- Mittasch, J.; Bottcher, C.; Frolov, A.; Strack, D.; Milkowski, C. Reprogramming the phenylpropanoid metabolism in seeds of oilseed rape by suppressing the orthologs of REDUCED EPIDERMAL FLUORESCENCE1. Plant Physiol. 2013, 161, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Eldin, M.G.S.; Kassem, H.; Fetouh, M.A.E. Metabolome classification of Brassica napus organs via UPLC-QTOF-PDA-MS and their anti-oxidant potential. Phytochem. Anal. 2013, 24, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kortesniemi, M.; Liu, P.Z.; Karonen, M.; Salminen, J.P. Analysis of hydrolysable tannins and other phenolic compounds in emblic leafflower (Phyllanthus emblica L.) fruits by high performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2012, 60, 8672–8683. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Jiang, J.; Ran, L.; Lu, C.; Wei, C.; Wang, Y. Analysis of flavonoids and hydroxycinnamic acid derivatives in rapeseeds (Brassica napus L. var. napus) by HPLC-PDA–ESI(−)-MSn/HRMS. J. Agric. Food Chem. 2014, 62, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kumar, K.; Anil, D.; Kapoor, H.C. Variations in antioxidant activity in broccoli (Brassica oleracea L.) cultivars. J. Food Biochem. 2007, 31, 621–638. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Phenolic composition, antioxidant capacity and antibacterial activity of selected Irish Brassica vegetables. Nat. Prod. Commun. 2011, 6, 1299–1304. [Google Scholar] [PubMed]
- Dubie, J.; Stancik, A.; Morra, M.; Nindo, C. Antioxidant extraction from mustard (Brassica juncea) seed meal using high-intensity ultrasound. J. Food Sci. 2013, 78, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Kim, D.O.; Padilla-Zakour, O.I.; Griffiths, P.D. Flavonoids and antioxidant capacity of various cabbage genotypes at juvenile stage. J. Food Sci. 2004, 69, 9–13. [Google Scholar] [CrossRef]
- The, S.S.; Bekhit, A.E.; Birch, J. Antioxidative polyphenols from defatted oilseed cakes: Effect of solvents. Antioxidants 2014, 3, 67–80. [Google Scholar]
- He, Q.; Zhang, Z.; Zhang, L. Anthocyanin accumulation, antioxidant ability and stability, and a transcriptional analysis of anthocyanin biosynthesis in purple heading Chinese cabbage (Brassica rapa L. ssp. pekinensis). J. Agric. Food Chem. 2016, 64, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant activity and phenolic composition of wild, edible, and medicinal fennel from different Mediterranean countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Qu, C.; Zhao, H.; Fu, F.; Wang, Z.; Zhang, K.; Zhou, Y.; Wang, X.; Wang, R.; Xu, X.; Tang, Z.; et al. Genome-wide survey of flavonoid biosynthesis genes and gene expression analysis between black- and yellow-seeded Brassica napus. Front. Plant Sci. 2016, 7, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the plant materials, for research only are available from the authors. |







| DPPH (%) | ABTS (%) | FRAP (μmoL Fe (II)/g DW) | ||||
|---|---|---|---|---|---|---|
| Black Seed | Yellow Seed | Black Seed | Yellow Seed | Black Seed | Yellow Seed | |
| 3 WAF | 18.12749 a | 1.593625 b | 38.36 a | 15.36 b | 246.48 a | 105.32 b |
| 4 WAF | 52.29084 a | 3.685259 b | 75.79 a | 21.79 b | 375.38 a | 112.29 b |
| 5 WAF | 78.18725 a | 28.68526 b | 98.36 a | 54.07 b | 432.52 a | 236.95 b |
| 6 WAF | 81.57371 a | 41.43426 b | 98.36 a | 75.50 b | 357.46 a | 274.08 b |
| Mature | 55.07968 b | 68.02789 a | 95.50 a | 98.07 a | 330.52 a | 313.55 b |
| Flavonoids | ABTS | DPPH | FRAP | |||||
|---|---|---|---|---|---|---|---|---|
| Black Seed | Yellow Seed | Black Seed | Yellow Seed | Black Seed | Yellow Seed | Black Seed | Yellow Seed | |
| Phenolics | 0.897 * | 0.8053 * | 0.535 | 0.695 | 0.713 | 0.607 | 0.921 * | 0.701 |
| Flavonoids | -- | -- | 0.747 | 0.936 * | 0.923 * | 0.889 * | 0.889 * | 0.948 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Meng, G.; Chen, S.; Chen, Y.; Jiang, J.; Wang, Y.-P. Correlation Analysis of Phenolic Contents and Antioxidation in Yellow- and Black-Seeded Brassica napus. Molecules 2018, 23, 1815. https://doi.org/10.3390/molecules23071815
Wang Y, Meng G, Chen S, Chen Y, Jiang J, Wang Y-P. Correlation Analysis of Phenolic Contents and Antioxidation in Yellow- and Black-Seeded Brassica napus. Molecules. 2018; 23(7):1815. https://doi.org/10.3390/molecules23071815
Chicago/Turabian StyleWang, Yue, Guisheng Meng, Sailing Chen, Yajie Chen, Jinjin Jiang, and You-Ping Wang. 2018. "Correlation Analysis of Phenolic Contents and Antioxidation in Yellow- and Black-Seeded Brassica napus" Molecules 23, no. 7: 1815. https://doi.org/10.3390/molecules23071815