Aromatic Thioacetal-Bridged ROS-Responsive Nanoparticles as Novel Gene Delivery Vehicles
Abstract
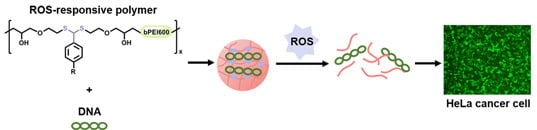
:1. Introduction
2. Results and Discussion
2.1. Polymer Synthesis and Characterization
2.2. Polymer Responds to Reactive Oxygen Species
2.3. Interaction with Plasmid DNA and Characterization of Polyplexes
2.4. In Vitro Gene Transfection
2.5. Cytotoxicity
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Title Polymers
3.2.1. Preparation of 2,2′-((4-(Trifluoromethyl)phenyl)methylene)bis(sulfanediyl))diethanol (Compound 2d)
3.2.2. Preparation of Diglycidyl Ether Linkers 3
3.2.3. Synthesis and Characterization of Target Polymers 4
3.3. Reactive Oxygen Species-Triggered Degradation of Thioketal Linkages
3.4. Agarose Gel Retardation
3.5. Particle Size and Zeta Potential Measurements
3.6. Reactive Oxygen Species-Responsive of Polyplexes
3.7. In Vitro Transfection Experiments
3.8. Cytotoxicity Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, H.; Cho, Y.-W.; Kang, S.-W.; Kwak, M.-K.; Huh, K.M.; Bae, Y.H.; Kang, H.C. Tempo-spatial activation of sequential quadruple stimuli for high gene expression of polymeric gene nanocomplexes. Mol. Pharm. 2017, 14, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Ilarduya, C.T.D.; Sun, Y.; Düzgüneş, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-T.; Chen, G.; Nie, X.; Wang, L.-H.; Ding, S.-G.; You, Y.-Z. Low generation PAMAM-based nanomicelles as ROS-responsive gene vectors with enhanced transfection efficacy and reduced cytotoxicity in vitro. New J. Chem. 2017, 41, 3273–3279. [Google Scholar] [CrossRef]
- Bouard, D.; Alazard-Dany, N.; Cosset, F.L. Viral vectors: From virology to transgene expression. Br. J. Pharmacol. 2009, 157, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Lu, H.; Li, W.; Zheng, Y.; Jiang, Z.; Zou, J.; Gao, H. Near-infrared triggered upconversion polymeric nanoparticles based on aggregation-induced emission and mitochondria targeting for photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 26731–26739. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Deng, W.; Goldys, E.M. Light-triggerable liposomes for enhanced endolysosomal escape and gene silencing in PC12 cells. Mol. Ther. Nucleic Acids 2017, 7, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-J.; He, D.; Kessel, E.; Padari, K.; Kempter, S.; Lächelt, U.; Rädler, J.O.; Pooga, M.; Wagner, E. Tumoral gene silencing by receptor-targeted combinatorial siRNA polyplexes. J. Control. Release 2016, 244 Pt B, 280–291. [Google Scholar] [CrossRef]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartono, S.B.; Phuoc, N.T.; Yu, M.; Jia, Z.; Monteiro, M.J.; Qiao, S.; Yu, C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B 2014, 2, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Rosariomeléndez, R.; Yu, W.; Uhrich, K.E. Biodegradable polyesters containing ibuprofen and naproxen as pendant groups. Biomacromolecules 2013, 14, 3542–3548. [Google Scholar] [CrossRef] [PubMed]
- Neu, M.; Fischer, D.; Kissel, T.J. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 2005, 7, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Xun, M.-M.; Zhang, J.-H.; Liu, Y.-H.; Zhang, J.; Xiao, Y.-P.; Guo, Q.; Li, S.; Yu, X.-Q. Polyethylenimine analogs for improved gene delivery: Effect of the type of amino groups. RSC Adv. 2016, 6, 5391–5400. [Google Scholar] [CrossRef]
- Li, H.; Jiang, H.; Zhao, M.; Fu, Y.; Sun, X. Intracellular redox potential-responsive micelles based on polyethylenimine-cystamine-poly(ε-caprolactone) block copolymer for enhanced miR-34a delivery. Polym. Chem. 2015, 6, 1952–1960. [Google Scholar] [CrossRef]
- Sarkar, K.; Debnath, M.; Kundu, P.P. Preparation of low toxic fluorescent chitosan-graft-polyethyleneimine copolymer for gene carrier. Carbohydr. Polym. 2013, 92, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Hirayama, A.; Nagasaki, Y. The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles. Biomaterials 2011, 32, 8021–8028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xun, M.-M.; Xiao, Y.-P.; Zhang, J.; Liu, Y.-H.; Peng, Q.; Guo, Q.; Wu, W.-X.; Xu, Y.; Yu, X.-Q. Low molecular weight PEI-based polycationic gene vectors via Michael addition polymerization with improved serum-tolerance. Polymer 2015, 65, 45–54. [Google Scholar] [CrossRef]
- Mao, C.Q.; Du, J.Z.; Sun, T.M.; Yao, Y.D.; Zhang, P.Z.; Song, E.W.; Wang, J. A biodegradable amphiphilic and cationic triblock copolymer for the delivery of siRNA targeting the acid ceramidase gene for cancer therapy. Biomaterials 2011, 32, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, W.; Jin, M.-J.; Fan, B.; Xia, G.-M.; Gao, Z.-G. Inhibition of murine breast cancer growth and metastasis by survivin-targeted siRNA using disulfide cross-linked linear PEI. Eur. J. Pharm. Sci. 2016, 82, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, C.J.; Liu, B. A photoactivatable AIE polymer for light-controlled gene delivery: Concurrent endo/lysosomal escape and DNA unpacking. Angew. Chem. Int. Ed. 2015, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.M.; Kim, H.; Park, D.; Kim, J.; Kim, W.J. Phenylboronic acid-sugar grafted polymer architecture as a dual stimuli-responsive gene carrier for targeted anti-angiogenic tumor therapy. Biomaterials 2016, 75, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, J.; Zhang, C.; Cao, Z.; Cheng, D.; Liu, J.; Shuai, X. Stimuli-responsive polymeric nanocarriers for efficient gene delivery. Top. Curr. Chem. 2017, 375, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Chen, C.; Tintaru, A.; Cao, Y.; Liu, J.; Ziarelli, F.; Tang, J.; Guo, H.; Rosas, R.; et al. A fluorinated bola-amphiphilic dendrimer for on-demand delivery of siRNA, via specific response to reactive oxygen species. Adv. Funct. Mater. 2016, 26, 8594–8603. [Google Scholar] [CrossRef]
- Shim, M.S.; Xia, Y. A reactive oxygen species (ROS)-responsive polymer for safe, efficient, and targeted gene delivery in cancer cells. Angew. Chem. Int. Ed. 2013, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, X.; Liu, L.; Zhang, Y.; Lu, Y.; Zhang, Y.; Chen, Q.; Ruan, C.; Guo, Q.; Li, C.; et al. Sequentially triggered nanoparticles with tumor penetration and intelligent drug release for pancreatic cancer therapy. Adv. Sci. 2018, 5, 1701070. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Rejinold, N.S.; Lee, D.; Jon, S.; Kim, Y.C. Protease-activatable cell-penetrating peptide possessing ROS-triggered phase transition for enhanced cancer therapy. J. Control. Release 2017, 264, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Chen, Y.; Dou, Y.; Tao, H.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Structure-property correlations of reactive oxygen species-responsive and hydrogen peroxide-eliminating materials with anti-oxidant and anti-inflammatory activities. Chem. Mater. 2017, 29, 8221–8238. [Google Scholar] [CrossRef]
- Lee, S.H.; Boire, T.C.; Lee, J.B.; Gupta, M.K.; Zachman, A.L.; Rath, R.; Sung, H.J. ROS-cleavable proline oligomer crosslinking of polycaprolactone for pro-angiogenic host response. J. Mater. Chem. B 2014, 2, 7109–7113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.-Y.; Su, G.-M.; Chen, C.-K.; Chiang, Y.-T.; Lo, C.-L. Specific cancer cytosolic drug delivery triggered by oxygen species-responsive micelles. Biomacromolecules 2016, 17, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-C.; Du, F.-S.; Li, Z.-C. Oxidation-responsive polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 3413–3426. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Zhong, Z. Bioresponsive polymeric nanotherapeutics for targeted cancer chemotherapy. Nano Today 2015, 10, 656–670. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Kim, J.; Kim, W.J. Reactive-oxygen-species-responsive drug delivery systems: Promises and challenges. Adv. Sci. 2017, 4, 1600124. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.Q.; Xing, L.; Cui, P.F.; Qiao, J.B.; He, Y.J.; Chen, B.A.; Jin, L.; Jiang, H.L. Curcumin-coordinated nanoparticles with improved stability for reactive oxygen species-responsive drug delivery in lung cancer therapy. Int. J. Nanomed. 2017, 12, 855–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, W.-J.; Yu, X.-C.; Wang, B.; Zhang, J.; Yu, Q.-Y.; Zhou, X.-D.; Yu, X.-Q. TACN-based oligomers with aromatic backbones for efficient nucleic acid delivery. Chem. Commun. 2014, 50, 6454–6457. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.-R.; Liu, Y.-H.; Zhang, J.; Yu, Q.-Y.; Huang, Z.; Wang, B.; Yu, X.-Q. Low molecular weight oligomers with aromatic backbone as efficient nonviral gene vectors. ACS Appl. Mater. Interfaces 2016, 8, 10743–10751. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, R.-C.; Yi, W.-J.; Zhao, Z.-G. Biodegradable poly(amino ester) with aromatic backbone as efficient nonviral gene delivery vectors. Molecules 2017, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.-J.; Zhang, Q.-F.; Zhang, J.; Liu, Q.; Ren, L.; Chen, Q.-M.; Guo, L.; Yu, X.-Q. Cyclen-based lepidic oligomers as potential gene delivery vehicles. Acta Biomater. 2014, 10, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-F.; Yu, Q.-Y.; Geng, Y.; Zhang, J.; Wu, W.-X.; Wang, G.; Gu, Z.; Yu, X.-Q. Ring-opening polymerization for hyperbranched polycationic gene delivery vectors with excellent serum tolerance. ACS Appl. Mater. Interfaces 2014, 6, 15733–15742. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, Y.-H.; Xun, M.-M.; Zhang, J.; Huang, Z.; Zhou, X.-D.; Yu, X.-Q. Diol glycidyl ether-bridged low molecular weight PEI as potential gene delivery vehicles. J. Mater. Chem. B 2015, 3, 2660–2670. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Domb, A.J. Cationic Polysaccharides for Gene Delivery. Mater. Sci. Eng. C 2007, 27, 595–598. [Google Scholar] [CrossRef]
- Liu, Y.M.; Reineke, T.M. Hydroxyl stereochemistry and amine number within Poly(glycoamidoamine)s affect intracellular DNA delivery. J. Am. Chem. Soc. 2005, 127, 3004–3015. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cheng, G.; Xie, L.; Nie, Y.; He, B.; Gu, Z. Polyethyleneimine/DNA polyplexes with reduction-sensitive hyaluronic acid derivatives shielding for targeted gene delivery. Biomaterials 2013, 34, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Laskar, R.A.; Begum, N.A.; Mir, M.H.; Rohman, M.R.; Khan, A.T. Nickel(II) chloride hexahydrate catalyzed reaction of aromatic aldehydes with 2-mercaptoethanol: Formation of supramolecular helical assemblage of the product. Tetrahedron Lett. 2013, 54, 5839–5844. [Google Scholar] [CrossRef]
Sample Availability: Not available. |







| Polymers | Mw (Da) | PDI |
|---|---|---|
| 4a | 9871 | 1.85 |
| 4b | 10,916 | 1.97 |
| 4c | 7123 | 1.70 |
| 4d | 8209 | 1.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.-Q.; Yi, W.-J.; Liu, Q.; Yang, X.-J.; Zhao, Z.-G. Aromatic Thioacetal-Bridged ROS-Responsive Nanoparticles as Novel Gene Delivery Vehicles. Molecules 2018, 23, 2061. https://doi.org/10.3390/molecules23082061
Lin G-Q, Yi W-J, Liu Q, Yang X-J, Zhao Z-G. Aromatic Thioacetal-Bridged ROS-Responsive Nanoparticles as Novel Gene Delivery Vehicles. Molecules. 2018; 23(8):2061. https://doi.org/10.3390/molecules23082061
Chicago/Turabian StyleLin, Guo-Qing, Wen-Jing Yi, Qiang Liu, Xue-Jun Yang, and Zhi-Gang Zhao. 2018. "Aromatic Thioacetal-Bridged ROS-Responsive Nanoparticles as Novel Gene Delivery Vehicles" Molecules 23, no. 8: 2061. https://doi.org/10.3390/molecules23082061