Antihyperalgesic Activity of Atomoxetine on Diabetes-Induced Neuropathic Pain: Contribution of Noradrenergic and Dopaminergic Systems
Abstract
:1. Introduction
2. Results
2.1. Beneficial Effects of Atomoxetine on Neuropathic Pain
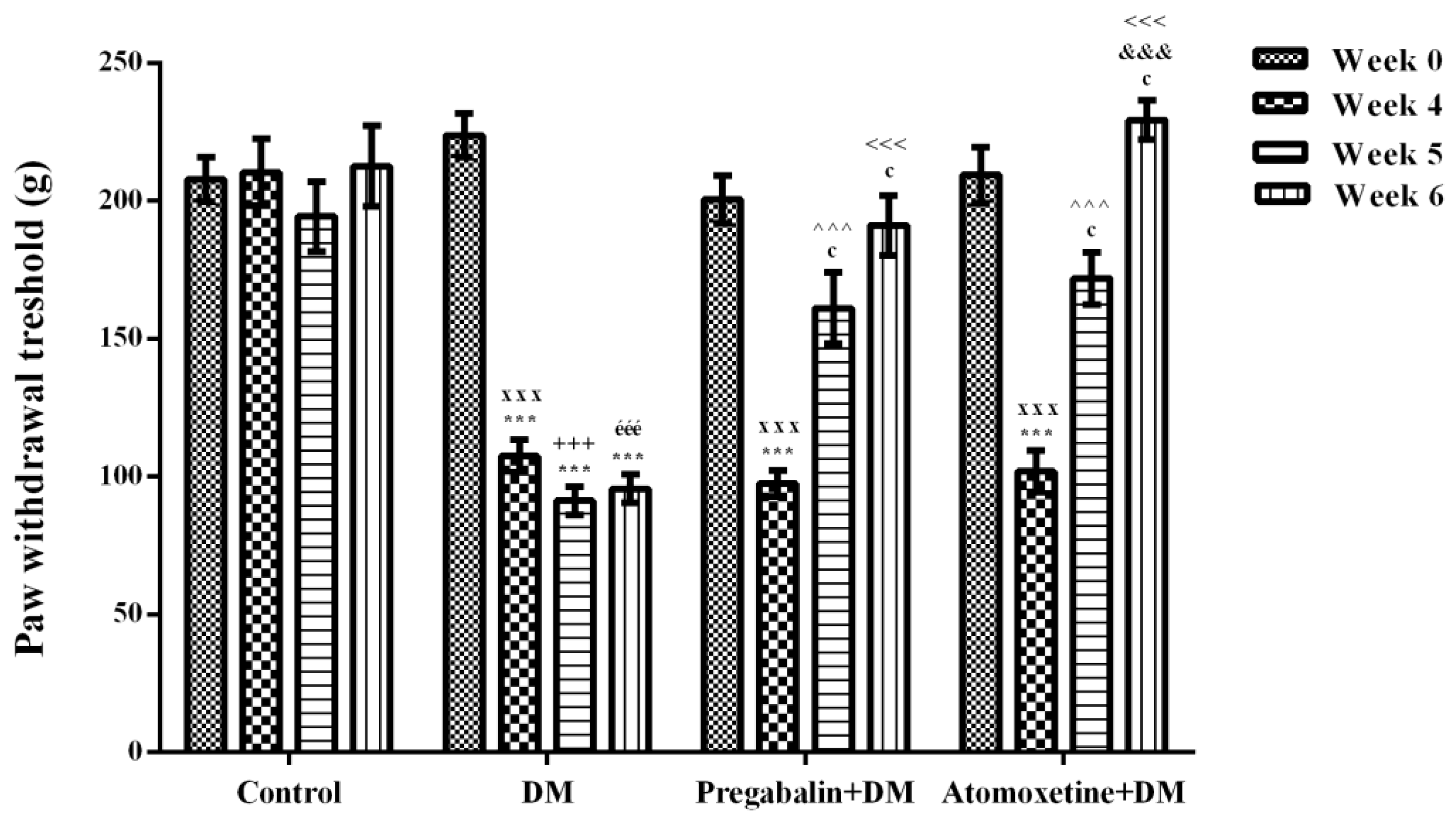
2.1.1. Atomoxetine Alleviates Diabetes-Induced Mechanical Hyperalgesia
2.1.2. Atomoxetine Alleviates Diabetes-Induced Thermal Hyperalgesia
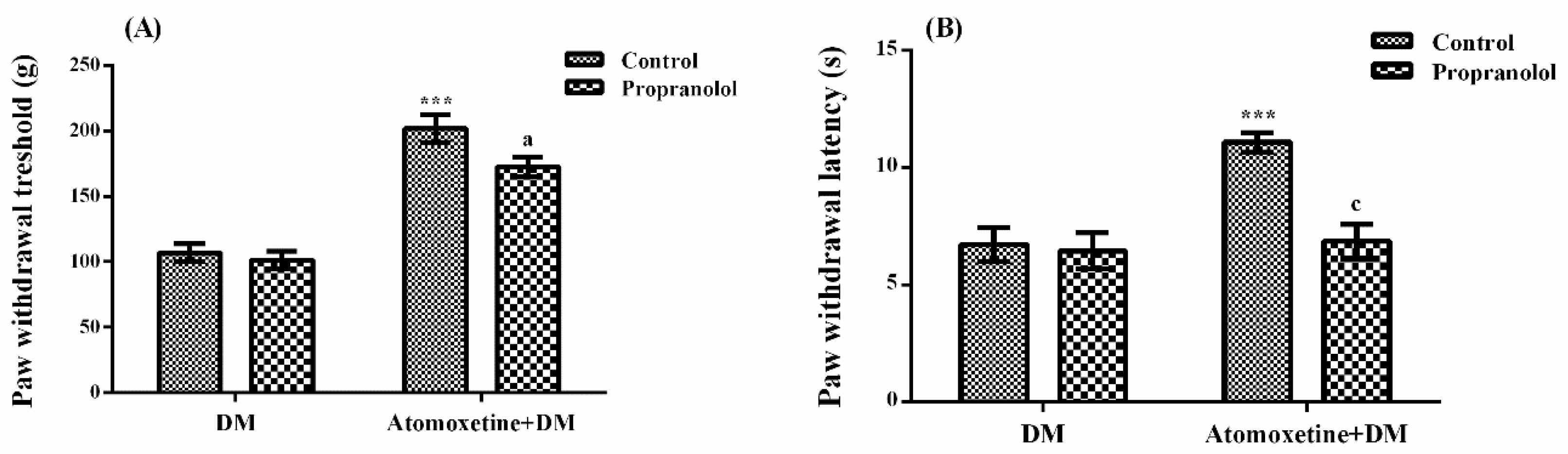
2.1.3. Catecholaminergic System Mediates the Beneficial Effect of Atomoxetine on Diabetes-Induced Mechanical and Thermal Hyperalgesia
2.2. Antihyperalgesic Efficacy of Atomoxetine is not Related to a Possible Alteration in the Motor Activity of Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Induction of Experimental Diabetes Model in Rats
4.4. Design of the Experimental Groups
4.5. Neuropathic Pain Tests
4.5.1. Randall-Selitto Test
4.5.2. Hargreaves Test (Plantar Test)
4.5.3. Mechanistic studies
4.6. Activity Cage Tests
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boulton, A.J.M.; Malik, R.A.; Arezzo, J.C.; Sosenko, J.M. Diabetic somatic neuropathies. Diabetes Care 2004, 27, 1458–1486. [Google Scholar] [CrossRef] [PubMed]
- Sing, R.; Kishore, L.; Kaur, N. Diabetic peripheral neuropathy: Current perspective and future directions. Pharmacol. Res. 2014, 80, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.S.; de Gray, L.; George, E. Painful diabetic neuropathy. Contin. Educ. Anaesth. Crit. Care Pain 2014, 14, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Berti-Mattera, L.N.; Kern, T.S.; Siegel, R.E.; Nemet, I.; Mitchell, R. Sulfasalazine blocks the development of tactile allodynia in diabetic rats. Diabetes 2008, 57, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis, Treatment and Follow-Up of Diabetic Neuropathy. Available online: https://www.deutsche-diabetes-gesellschaft.de/fileadmin/Redakteur/Leitlinien/Englische_Leitlinien/GUIDELINE_DIABETIC_NEUROPATHY_05_2004_DDG_01_2006.pdf (accessed on 18 July 2018).
- Javed, S.; Petropoulos, I.N.; Alam, U.; Malik, R.A. Treatment of painful diabetic neuropathy. Ther. Adv. Chronic Dis. 2015, 6, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Fedder, D.; Saadabadi, A. Atomoxetine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493234/ (accessed on 14 August 2018).
- Hiroki, T.; Suto, T.; Saito, S.; Obata, H. Repeated administration of amitriptyline in neuropathic pain: Modulation of the noradrenergic descending inhibitory system. Anesth. Analg. 2017, 125, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, H.; Obata, H.; Saito, S. Antihyperalgesic effect of duloxetine and amitriptyline in rats after peripheral nerve injury: Influence of descending noradrenergic plasticity. Neurosci. Lett. 2015, 602, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Suto, T.; Saito, S.; Obata, H. Repeated administration of duloxetine suppresses neuropathic pain by accumulating effects of noradrenaline in the spinal cord. Anesth. Analg. 2018, 126, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Analgesic mechanisms of antidepressants for neuropathic pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef] [PubMed]
- Aydın, T.H.; Can, Ö.D.; Demir Özkay, Ü.; Turan, N. Effect of subacute agomelatine treatment on painful diabetic neuropathy: Involvement of catecholaminergic mechanisms. Fundam. Clin. Pharmacol. 2016, 30, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Üçel, U.İ.; Can, Ö.D.; Demir Özkay, Ü.; Öztürk, Y. Antihyperalgesic and antiallodynic effects of mianserin on diabetic neuropathic pain: a study on mechanism of action. Eur. J. Pharmacol. 2015, 756, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Onal, A.; Parlar, A.; Ulker, S. Milnacipran attenuates hyperalgesia and potentiates antihyperalgesic effect of tramadol in rats with mononeuropathic pain. Pharmacol. Biochem. Behav. 2007, 88, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Widerlöv, E.; Lewander, T. Inhibition of the in vivo biosynthesis and changes of catecholamine levels in rat brain after alpha-methyl-p-tyrosine; time-and dose-response relationships. Naunyn. Schm. Arch. Pharmacol. 1978, 304, 111–123. [Google Scholar] [CrossRef]
- Corrodi, H.; Hanson, L.C. Central effects of an inhibitör of tyrosine hydroxylation. Psychopharmacologia 1966, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Malcangio, M.; Tomlinson, D.R. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain 1998, 76, 151–157. [Google Scholar] [CrossRef]
- Pertovaara, A. The noradrenergic pain regulation system: A potential target for pain therapy. Eur. J. Pharmacol. 2013, 716, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.K. Spinal inhibitory neurotransmission in neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.X.; Xu, F.Y.; Xu, W.J.; Zhao, Y.; Qu, C.L.; Tang, J.S.; Barry, D.M.; Du, J.Q.; Huo, F.Q. The role of α2 adrenoceptor in mediating noradrenaline action in the ventrolateralorbital cortex on allodynia following spared nerve injury. Exp. Neurol. 2013, 248, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.E.; Ciszek, B.P.; Nackley, A.G. β2-and β3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines. Pain 2014, 155, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hartung, J.E.; Bortsov, A.V.; Kim, S.; O'Buckley, S.C.; Kozlowski, J.; Nackley, A.G. Sustained stimulation of β2- and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav. Immun. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.; Khoshnoud, M.J.; Taghian, M.; Aliasgharzadeh, M.; Mesdaghinia, A. Quetiapine reverses paclitaxel-induced neuropathic pain in mice: Role of alpha2-adrenergic receptors. Iran J. Basic Med. Sci. 2017, 20, 1182–1188. [Google Scholar] [PubMed]
- Chenaf, C.; Chapuy, E.; Libert, F.; Marchand, F.; Courteix, C.; Bertrand, M.; Gabriel, C.; Mocaër, E.; Eschalier, A.; Authier, N. Agomelatine: A new opportunity to reduce neuropathic pain-preclinical evidence. Pain 2017, 158, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Crocetti, L.; Giovannoni, M.P.; Vergelli, C.; Ghelardini, C. α2 adrenoceptor: A target for neuropathic pain treatment. Mini Rev. Med. Chem. 2017, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Chung, Y.; Choi, S.; Min, B.I.; Kim, S.K. Duloxetine protects against oxaliplatin-induced neuropathic pain and spinal neuron hyperexcitability in rodents. Int. J. Mol. Sci. 2017, 18, 2626. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhu, Y.M.; Zhang, Y.X.; Liang, F.; Jia, H.; Qu, C.L.; Wang, J.; Tang, J.S.; Lu, S.M.; Huo, F.Q.; Yan, C.X. Activation of α1 adrenoceptors in ventrolateral orbital cortex attenuates allodynia induced by spared nerve injury in rats. Neurochem. Int. 2016, 99, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Choucair-Jaafar, N.; Salvat, E.; Freund-Mercier, M.J.; Barrot, M. The antiallodynic action of nortriptyline and terbutaline is mediated by β(2) adrenoceptors and δ opioid receptors in the ob/ob model of diabetic polyneuropathy. Brain Res. 2014, 1546, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Choucair-Jaafar, N.; Benbouzid, M.; Tessier, L.H.; Muller, A.; Hein, L.; Freund-Mercier, M.J.; Barrot, M. Beta(2)-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann. Neurol. 2009, 65, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Tessier, L.H.; Petit-Demoulière, N.; Doridot, S.; Hein, L.; Freund-Mercier, M.J.; Barrot, M. β2-adrenoceptors are essential for desipramine, venlafaxine or reboxetine action in neuropathic pain. Neurobiol. Dis. 2009, 33, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Abdul Rahim, M.H.; Roosli, R.A.J.; Mohd Sani, M.H.; Omar, M.H.; Mohd Tohid, S.F.; Othman, F.; Ching, S.M.; Abdul Kadir, A. Antinociceptive activity of methanolic extract of Clinacanthus nutans leaves: Possible mechanisms of action involved. Pain Res. Manag. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Morioka, N.; Abe, H.; Fujii, S.; Miyauchi, K.; Nakamura, Y.; Hisaoka-Nakashima, K.; Nakata, Y. Stimulation of spinal dorsal horn β2-adrenergic receptor ameliorates neuropathic mechanical hypersensitivity through a reduction of phosphorylation of microglial p38 MAP kinase and astrocytic c-jun N-terminal kinase. Neurochem. Int. 2016, 101, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Grignon, S.; Marchand, S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse 2009, 63, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood-Walker, S.M.; Hope, P.J.; Mitchell, R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurones. J. Physiol. 1988, 399, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, W.; Nakatsuka, T.; Miyazaki, N.; Yamada, H.; Takeda, D.; Fujita, T.; Kumamoto, E.; Yoshida, M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain 2011, 152, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Yazdnian, M.; Haghparast, A. Role of dopamine, D2-like receptors within the ventral tegmental area and nucleus accumbens in antinociception induced by lateral hypothalamus stimulation. Behav. Brain Res. 2015, 292, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.K.; Joshi, C.; Uppal, H. Stimulation of dopamine, D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res. 2003, 987, 135–143. [Google Scholar] [CrossRef]
- Chen, M.; Hoshino, H.; Saito, S.; Yang, Y.; Obata, H. Spinal dopaminergic involvement in the antihyperalgesic effect of antidepressants in a rat model of neuropathic pain. Neurosci. Lett. 2017, 649, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sindrup, S.H.; Otto, M.; Finnerup, N.B.; Jensen, T.S. Antidepressants in the treatment of neuropathic pain. Basic Clin. Pharmacol. Toxicol. 2005, 96, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Badescu, S.V.; Tataru, C.P.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zagrean, A.M.; Zagrean, L. Effects of caffeine on locomotor activity in streptozotocin-induced diabetic rats. J. Med. Life 2016, 9, 275–279. [Google Scholar] [PubMed]
- Fox, A.; Eastwood, C.; Gentry, C.; Manning, D.; Urban, L. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain 1999, 81, 307–316. [Google Scholar] [CrossRef]
- Kou, Z.Z.; Li, C.Y.; Hu, J.C.; Yin, J.B.; Zhang, D.L.; Liao, Y.H.; Wu, Z.Y.; Ding, T.; Qu, J.; Li, H.; et al. Alterations in the neural circuits from peripheral afferents to the spinal cord: possible implications for diabetic polyneuropathy in streptozotocin-induced type 1 diabetic rats. Front. Neural. Circuits 2014, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, D.; Yu, X.; Xie, X.; Wang, L.; Lian, L.; Fei, N.; Chen, J.; Zhu, N.; Wang, G.; et al. The antinociceptive effects of ferulic acid on neuropathic pain: Involvement of descending monoaminergic system and opioid receptors. Oncotarget 2016, 7, 20455–20468. [Google Scholar] [CrossRef] [PubMed]
- Field, M.J.; McCleary, S.; Hughes, J.; Singh, L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain 1999, 80, 391–398. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimoshige, Y.; Yamaji, T.; Murai, N.; Aoki, T.; Matsuoka, N. Pharmacological characterization of standard analgesics on mechanical allodynia in streptozotocin-induced diabetic rats. Neuropharmacology 2009, 57, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Perry, K.W.; Koch-Krueger, S.; Katner, J.; Svensson, K.A.; Bymaster, F.P. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology 2006, 50, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.E.; Yuan, W.; Lou, X.; Zhu, T. Streptozotocin-induced diabetic hyperalgesia in rats is associated with upregulation of toll-like receptor 4 expression. Neurosci. Lett. 2012, 526, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Bordet, T.; Buisson, B.; Michaud, M.; Abitbol, J.L.; Marchand, F.; Grist, J.; Andriambeloson, E.; Malcangio, M.; Pruss, R.M. Specific antinociceptive activity of cholest-4-en-3-one, oxime (TRO19622) in experimental models of painful diabetic and chemotherapy-induced neuropathy. J. Pharmacol. Exp. Ther. 2008, 326, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Hara, K.; Haranishi, Y.; Sata, T. Antinociceptive effect of intrathecal administration of taurine in rat models of neuropathic pain. Can. J. Anaesth. 2011, 58, 630–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soulage, C.; Perrin, D.; Cottet-Emard, J.M.; Pequignot, J.; Dalmaz, Y.; Pequignot, J.M. Central and peripheral changes in catecholamine biosynthesis and turnover in rats after a short period of ozone exposure. Neurochem. Int. 2004, 45, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Forman, L.J. NMDA receptor antagonism produces antinociception which is partially mediated by brain opioids and dopamine. Life Sci. 1999, 64, 1877–1887. [Google Scholar] [CrossRef]
- Safieh-Garabedian, B.; Poole, S.; Haddad, J.J.; Massaad, C.A.; Jabbur, S.J.; Saadé, N.E. The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology 2002, 42, 864–872. [Google Scholar] [CrossRef]
- Can, O.D.; Oztürk, Y.; Ozkay, U.D. Effects of insulin and St. John’s wort treatments on anxiety, locomotory activity, depression, and active learning parameters of streptozotocin-diabetic rats. Planta Med. 2011, 77, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Pirondi, S.; Kuteeva, E.; Giardino, L.; Ferraro, L.; Antonelli, T.; Bartfai, T.; Ogren, S.O.; Hokfelt, T.; Calza, L. Behavioral and neurochemical studies on brain aging in galanin overexpressing mice. Neuropeptides 2005, 39, 305–312. [Google Scholar] [CrossRef] [PubMed]






| Experimental Groups | Treatment | |
|---|---|---|
| Day 0 | Days 28–42 | |
| Control group | Citrate buffer, i.v. | Physiological saline, p.o. |
| DM group | STZ, i.v. | Physiological saline, p.o. |
| Pregabalin + DM group | STZ, i.v. | Pregabalin (10 mg/kg, p.o) [45,46] |
| Atomoxetine + DM group | STZ, i.v. | Atomoxetine (3 mg/kg, i.p.) [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbaros, M.B.; Can, Ö.D.; Üçel, U.İ.; Turan Yücel, N.; Demir Özkay, Ü. Antihyperalgesic Activity of Atomoxetine on Diabetes-Induced Neuropathic Pain: Contribution of Noradrenergic and Dopaminergic Systems. Molecules 2018, 23, 2072. https://doi.org/10.3390/molecules23082072
Barbaros MB, Can ÖD, Üçel Uİ, Turan Yücel N, Demir Özkay Ü. Antihyperalgesic Activity of Atomoxetine on Diabetes-Induced Neuropathic Pain: Contribution of Noradrenergic and Dopaminergic Systems. Molecules. 2018; 23(8):2072. https://doi.org/10.3390/molecules23082072
Chicago/Turabian StyleBarbaros, Mustafa Burak, Özgür Devrim Can, Umut İrfan Üçel, Nazlı Turan Yücel, and Ümide Demir Özkay. 2018. "Antihyperalgesic Activity of Atomoxetine on Diabetes-Induced Neuropathic Pain: Contribution of Noradrenergic and Dopaminergic Systems" Molecules 23, no. 8: 2072. https://doi.org/10.3390/molecules23082072





