Resveratrol Suppresses Matrix Metalloproteinase-2 Activation Induced by Lipopolysaccharide in Mouse Osteoblasts via Interactions with AMP-Activated Protein Kinase and Suppressor of Cytokine Signaling 1
Abstract
:1. Introduction
2. Results
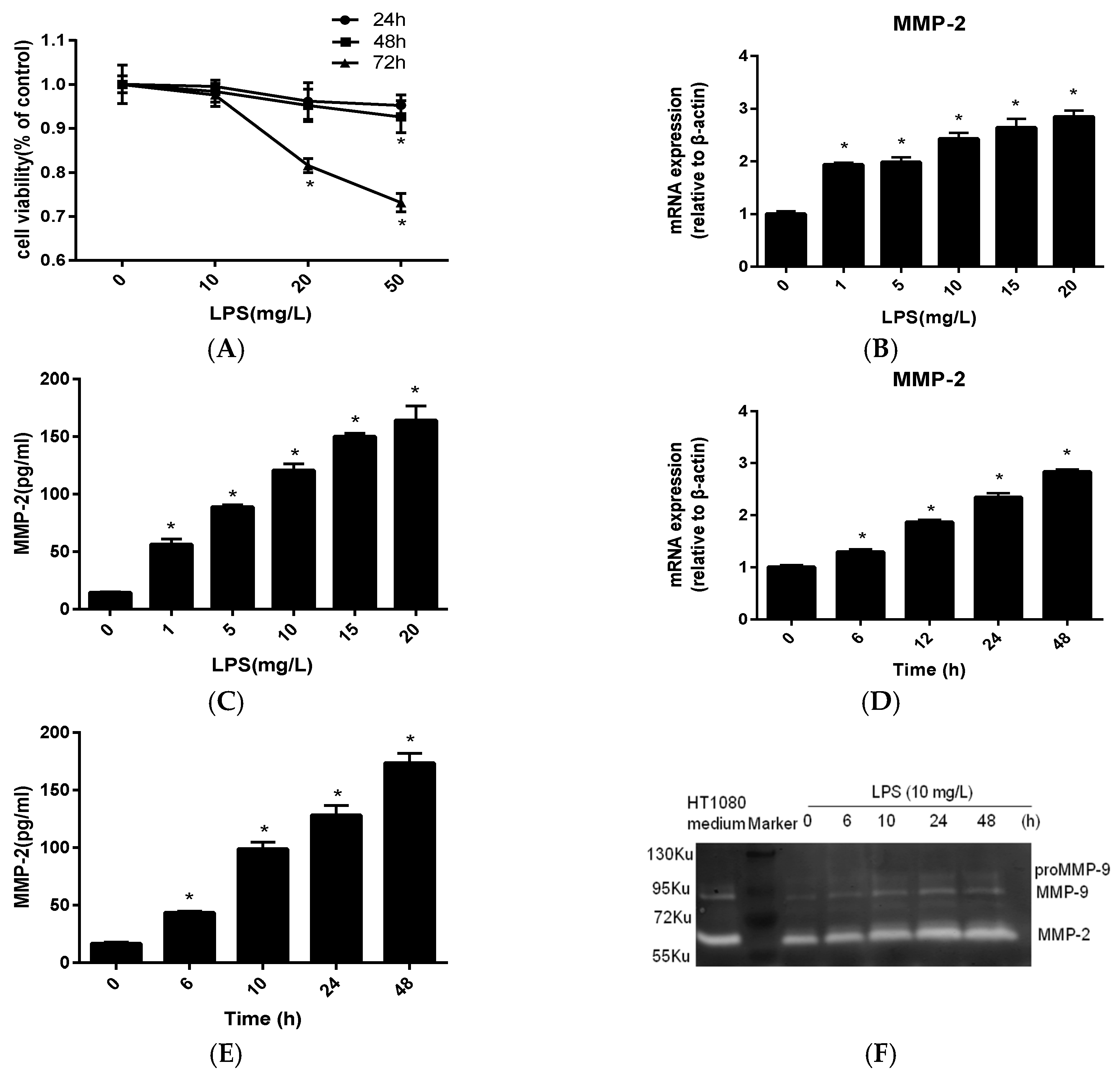
2.1. MMP-2 Is Elevated in MC3T3-E1 Cells by P. endodontalis LPS Treatment
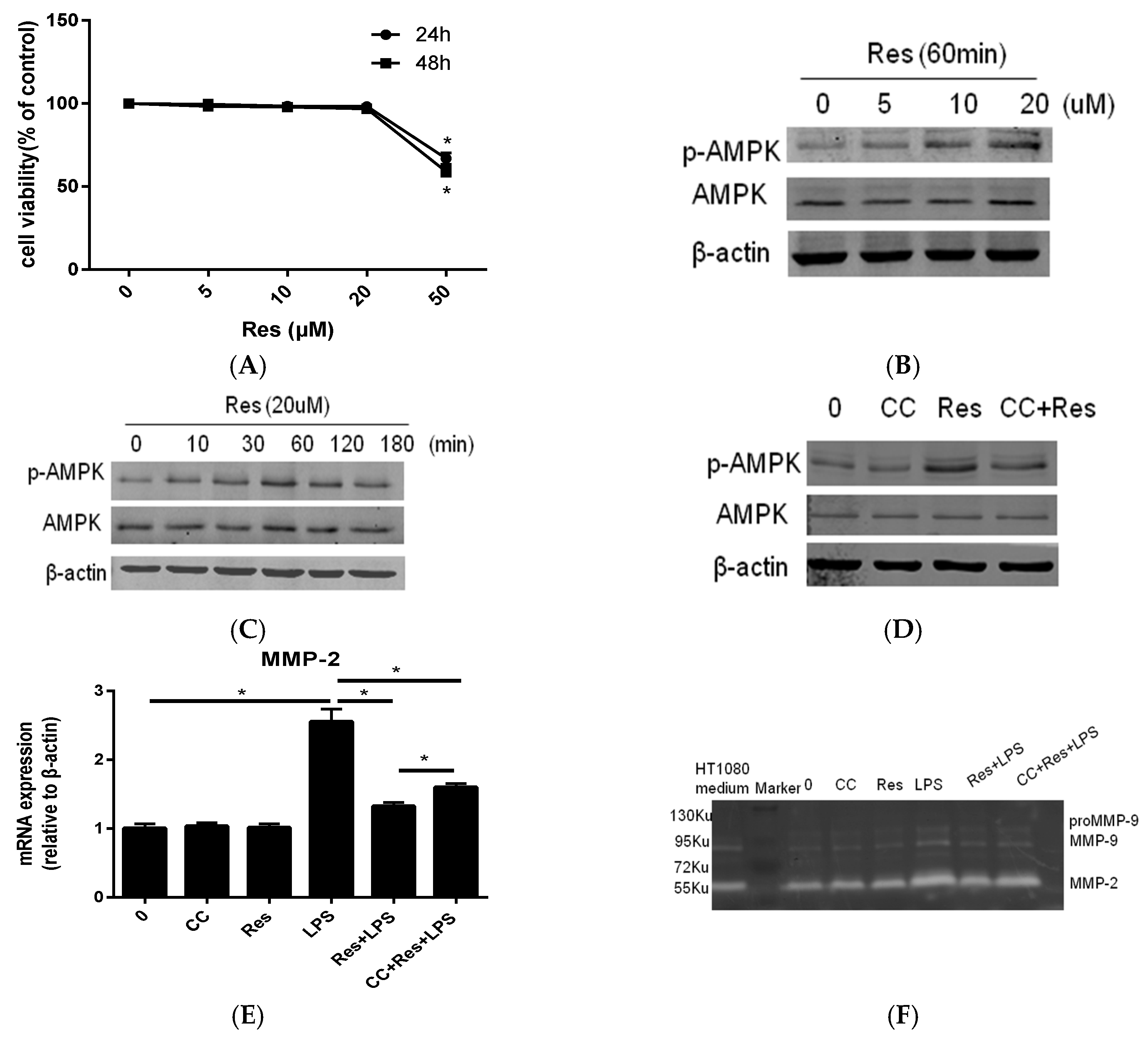
2.2. Resveratrol Inhibit P. endodontalis LPS-Stimulated MMP-2 Expression by Activating AMPK in MC3T3-E1 Cells
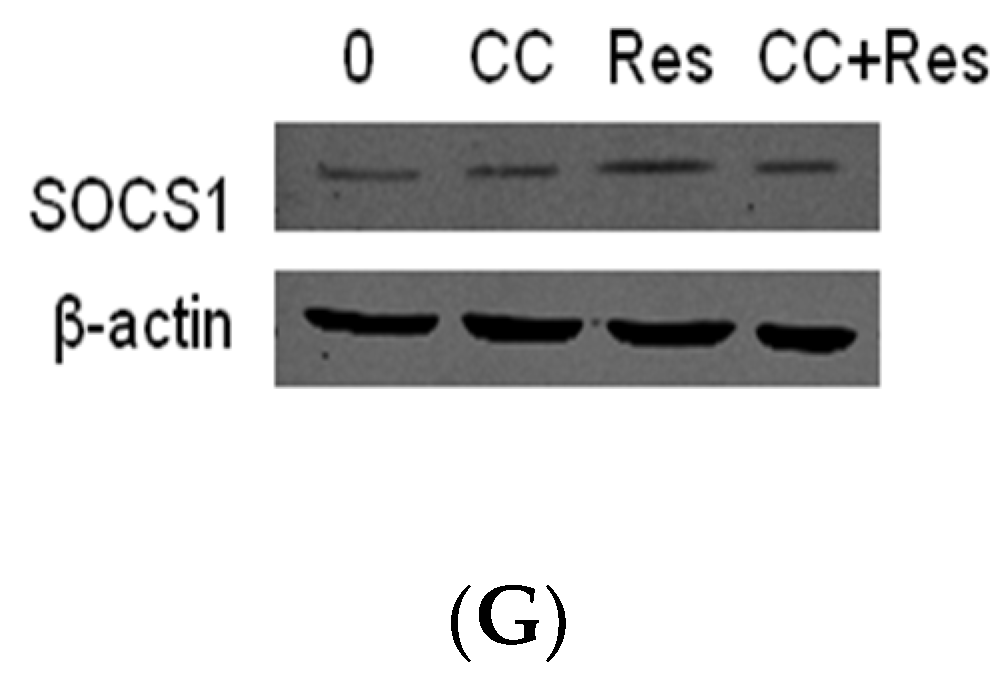
2.3. SOCS1 Was Involved in the Inhibitory Effects of Resveratrol on MMP-2 Expression in MC3T3-E1 Cells Stimualted by P. endodontalis LPS
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Bacterial Culture and LPS Extraction
4.3. MTT Assay
4.4. ELISA Analysis
4.5. Gelatin Zymography
4.6. Transfection
4.7. RNA Preparation and Real-Time PCR
4.8. Western Blotting
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Liljestrand, J.M.; Mantyla, P.; Paju, S.; Buhlin, K.; Kopra, K.A.; Persson, G.R.; Hernandez, M.; Nieminen, M.S.; Sinisalo, J.; Tjaderhane, L.; et al. Association of Endodontic Lesions with Coronary Artery Disease. J. Dent. Res. 2016, 95, 1358–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocas, I.N.; Siqueira, J.F., Jr.; Debelian, G.J. Analysis of symptomatic and asymptomatic primary root canal infections in adult Norwegian patients. J. Endod. 2011, 37, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Montagner, F.; Jacinto, R.C.; Signoretti, F.G.; Sanches, P.F.; Gomes, B.P. Clustering behavior in microbial communities from acute endodontic infections. J. Endod. 2012, 38, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.C.; Martinho, F.C.; Leite, F.R.; Nascimento, G.G.; Gomes, B.P. Proinflammatory Activity of Primarily Infected Endodontic Content against Macrophages after Different Phases of the Root Canal Therapy. J. Endod. 2015, 41, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, D.; Okamura, H.; Teramachi, J.; Ochiai, K.; Qiu, L.; Haneji, T. Calcium hydroxide suppresses Porphyromonas endodontalis lipopolysaccharide-induced bone destruction. J. Dent. Res. 2014, 93, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qiu, L.; Guo, J.; Yang, D.; Qu, L.; Yu, J.; Zhan, F.; Xue, M.; Zhong, M. TRIB3 mediates the expression of Wnt5a and activation of nuclear factor-kappaB in Porphyromonas endodontalis lipopolysaccharide-treated osteoblasts. Mol. Oral Microbiol. 2015, 30, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yu, Y.; Qiu, L.; Yang, D.; Yan, L.; Guo, J.; Jahan, R. Sirtuin 1 regulates matrix metalloproteinase-13 expression induced by Porphyromonas endodontalis lipopolysaccharide via targeting nuclear factor-kappaB in osteoblasts. J. Oral Microbiol. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.R.; Timo, S.; Claudia, B.; Marcela, H. Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.C. Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone 2011, 48, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Takahama, A., Jr.; Rocas, I.N.; Faustino, I.S.P.; Alves, F.R.F.; Azevedo, R.S.; Gomes, C.C.; Araujo-Filho, W.R.; Siqueira, J.F., Jr. Association between bacteria occurring in the apical canal system and expression of bone-resorbing mediators and matrix metalloproteinases in apical periodontitis. Int. Endod. J. 2018, 51, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Pereira Faustino, I.S.; Azevedo, R.S.; Takahama, A., Jr. Metalloproteinases 2 and 9 immunoexpression in periapical lesions from primary endodontic infection: possible relationship with the histopathological diagnosis and the presence of pain. J. Endod. 2016, 42, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Martinho, F.C.; Teixeira, F.F.; Cardoso, F.G.; Ferreira, N.S.; Nascimento, G.G.; Carvalho, C.A.; Valera, M.C. Clinical investigation of matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase complexes and their networks in apical periodontitis. J. Endod. 2016, 42, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4′-trihydroxy-stilbene) by grape berries in different developmental stages. Am. J. Enol. Viticult. 1991, 42, 41–46. [Google Scholar]
- Jeandet, P.; Breuil, A.C.; Adrian, M.; Weston, L.A.; Debord, S.; Meunier, P.; Maume, G.; Bessis, R. HPLC analysis of grapevine phytoalexins coupling photodiode array detection and fluorometry. Anal. Chem. 1997, 69, 5172–5177. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunier, P.; Peyron, D.; Trollat, P. Effect of enological practices on the resveratrol isomer content of wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.; Cheng, Y.C. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem. Int. 2018, 115, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.; Wahl, C.; Feldmann, M.; Jungck, D.; Strauch, J.; Stoelben, E.; Koch, A. Resveratrol attenuates the release of inflammatory cytokines from human bronchial smooth muscle cells exposed to lipoteichoic acid in chronic obstructive pulmonary disease. Basic Clin. Pharmacol. Toxicol. 2014, 114, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Cristina Orihuela-Campos, R.; Inagaki, Y.; Fukui, M.; Nagata, T.; Ito, H.O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014, 75, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Casati, M.Z.; Algayer, C.; Cardoso da Cruz, G.; Ribeiro, F.V.; Casarin, R.C.; Pimentel, S.P.; Cirano, F.R. Resveratrol decreases periodontal breakdown and modulates local levels of cytokines during periodontitis in rats. J. Periodontol. 2013, 84, e58–e64. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Andreelli, F.; Jorgensen, S.B.; Perrin, C.; Geloen, A.; Flamez, D.; Mu, J.; Lenzner, C.; Baud, O.; Bennoun, M.; et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Investig. 2003, 111, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Liau, N.P.D.; Laktyushin, A.; Lucet, I.S.; Murphy, J.M.; Yao, S.; Whitlock, E.; Callaghan, K.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018, 9, 1558. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xie, M.; Wang, X.; Jiang, X.; Li, J.; Huang, H. miR-155 modulates TNF-alpha-inhibited osteogenic differentiation by targeting SOCS1 expression. Bone 2012, 51, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Kantarci, A.; Herrera, B.S.; Gao, H.; Van Dyke, T.E. A critical role for suppressors of cytokine signaling 3 in regulating LPS-induced transcriptional activation of matrix metalloproteinase-13 in osteoblasts. Peer J. 2013, 1, e51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Wang, Y.; Shen, A.; Cai, W. Resveratrol upregulates SOCS1 production by lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting miR-155. Int. J. Mol. Med. 2017, 39, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Dragone, T.; Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Panaro, M.A. Resveratrol counteracts lipopolysaccharide-mediated microglial inflammation by modulating a SOCS-1 dependent signaling pathway. Toxicol. In Vitro 2014, 28, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Tat, S.K.; Pelletier, J.P.; Mineau, F.; Caron, J.; Martel-Pelletier, J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone 2011, 49, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Pattamapun, K.; Handagoon, S.; Sastraruji, T.; Gutmann, J.L.; Pavasant, P.; Krisanaprakornkit, S. Decreased levels of matrix metalloproteinase-2 in root-canal exudates during root canal treatment. Arch. Oral Biol. 2017, 82, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S.B.; Hakki, S.S.; Hakki, E.E.; Durak, Y.; Kantarci, A. Porphyromonas gingivalis Lipopolysaccharide Induces a Pro-inflammatory Human Gingival Fibroblast Phenotype. Inflammation 2017, 40, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.; Ham, S.A.; Hwang, J.S.; Lee, W.J.; Paek, K.S.; Oh, J.W.; Kim, J.H.; Do, J.T.; Han, C.W.; Kim, J.H.; et al. Peroxisome proliferator-activated receptor delta inhibits Porphyromonas gingivalis lipopolysaccharide-induced activation of matrix metalloproteinase-2 by downregulating NADPH oxidase 4 in human gingival fibroblasts. Mol. Oral Microbiol. 2016, 31, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.A.; Kuo, C.H.; Chen, B.Y.; Li, Y.; Liu, Y.C.; Chen, J.H.; Shieh, C.J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, C.; Zhang, J.; Liu, B.; Du, Q. Resveratrol inhibits inflammation and ameliorates insulin resistant endothelial dysfunction via regulation of AMP-activated protein kinase and sirtuin 1 activities. J. Diabetes 2016, 8, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Weikel, K.A.; Cacicedo, J.M.; Ido, Y. Resveratrol-induced amp-activated protein kinase activation is cell-type dependent: Lessons from basic research for clinical application. Nutrients 2017, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Li, W.D.; Li, N.P.; Song, D.D.; Rong, J.J.; Qian, A.M.; Li, X.Q. Metformin inhibits endothelial progenitor cell migration by decreasing matrix metalloproteinases, MMP-2 and MMP-9, via the AMPK/mTOR/autophagy pathway. Int. J. Mol. Med. 2017, 39, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Um, J.Y.; Lee, S.A.; Park, J.H.; Shin, J.M.; Park, I.H.; Lee, H.M. Role of adenosine monophosphate-activated protein kinase on cell migration, matrix contraction, and matrix metalloproteinase-1 and matrix metalloproteinase-2 production in nasal polyp-derived fibroblasts. Am. J. Rhinol. Allergy 2017, 31, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Alexander, W.S.; Hilton, D.J. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol 2004, 22, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Guenterberg, K.D.; Lesinski, G.B.; Mundy-Bosse, B.L.; Karpa, V.I.; Jaime-Ramirez, A.C.; Wei, L.; Carson, W.E. Enhanced anti-tumor activity of interferon-alpha in SOCS1-deficient mice is mediated by CD4(+) and CD8(+) T cells. Cancer Immunol. Immunother. 2011, 60, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Garlet, T.P.; Trombone, A.P.; Repeke, C.E.; Letra, A.; Granjeiro, J.M.; Campanelli, A.P.; Garlet, G.P. The potential role of suppressors of cytokine signaling in the attenuation of inflammatory reaction and alveolar bone loss associated with apical periodontitis. J. Endod. 2008, 34, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.W.; Lee, J.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, G.; Shukla, S.; Srivastava, Y.; Masaldan, S.; Mehta, S.; Bhambhani, S.; Sharma, S.; Mehrotra, R.; Das, B.C.; Bharti, A.C. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signaling and downstream effects during cervical carcinogenesis. Mol. Cancer 2015, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, Y.; Dong, R.; Dai, R.; Zhou, M. Suppressor of cytokine signaling 1-modulated metalloproteinases and tissue inhibitor of metalloproteinase in pulmonary fibrosis. Mol. Med. Rep. 2015, 12, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xin, H.; Tang, W.; Li, Y.; Zhang, Z.; Fan, L.; Miao, L.; Tan, B.; Wang, X.; Zhu, Y.Z. AMPK serves as a therapeutic target against anemia of inflammation. Antioxid. Redox Signal. 2017, 27, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhu, L.; Hong, X.; Wang, Y.T.; Wang, F.; Bao, J.P.; Xie, X.H.; Liu, L.; Wu, X.T. Resveratrol attenuated TNF-alpha-induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp. Biol. Med. 2016, 241, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, L.; Liu, X.; Lu, T.; Xie, C.; Jia, J. Resveratrol attenuates microglial activation via SIRT1-SOCS1 pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8791832. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Moseley, R.; Sloan, A.J.; Youde, S.J.; Waddington, R.J. Lipopolysaccharide alters decorin and biglycan synthesis in rat alveolar bone osteoblasts: Consequences for bone repair during periodontal disease. Eur. J. Oral Sci. 2008, 116, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Meng, F.; Chen, Z.; Tomlinson, B.N.; Wesley, J.M.; Sun, G.Y.; Whaley-Connell, A.T.; Sowers, J.R.; Cui, J.; Gu, Z. Two-dimensional zymography differentiates gelatinase isoforms in stimulated microglial cells and in brain tissues of acute brain injuries. PLoS ONE 2015, 10, e0123852. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Li, X.; Mi, J.; Qu, L.; Yang, D.; Guo, J.; Qiu, L. Resveratrol Suppresses Matrix Metalloproteinase-2 Activation Induced by Lipopolysaccharide in Mouse Osteoblasts via Interactions with AMP-Activated Protein Kinase and Suppressor of Cytokine Signaling 1. Molecules 2018, 23, 2327. https://doi.org/10.3390/molecules23092327
Yu Y, Li X, Mi J, Qu L, Yang D, Guo J, Qiu L. Resveratrol Suppresses Matrix Metalloproteinase-2 Activation Induced by Lipopolysaccharide in Mouse Osteoblasts via Interactions with AMP-Activated Protein Kinase and Suppressor of Cytokine Signaling 1. Molecules. 2018; 23(9):2327. https://doi.org/10.3390/molecules23092327
Chicago/Turabian StyleYu, Yaqiong, Xiaolin Li, Jing Mi, Liu Qu, Di Yang, Jiajie Guo, and Lihong Qiu. 2018. "Resveratrol Suppresses Matrix Metalloproteinase-2 Activation Induced by Lipopolysaccharide in Mouse Osteoblasts via Interactions with AMP-Activated Protein Kinase and Suppressor of Cytokine Signaling 1" Molecules 23, no. 9: 2327. https://doi.org/10.3390/molecules23092327




