Natural Peptides in Drug Discovery Targeting Acetylcholinesterase
Abstract
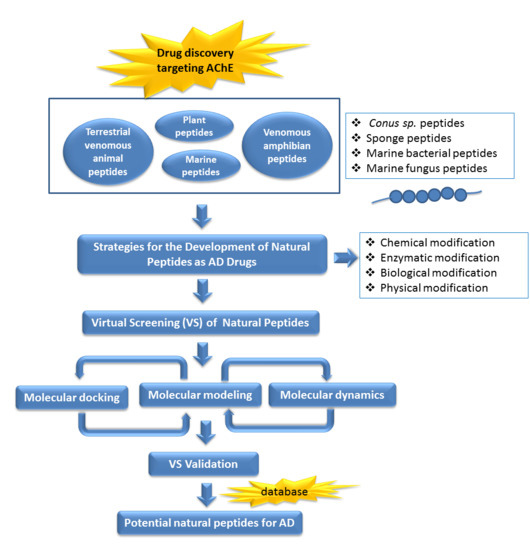
:1. Introduction
2. Neuropeptides from Plant Sources
3. Neuropeptides from Marine Sources
3.1. Cone Snail Conotoxins
3.2. AChEIs from Sponges
3.3. Marine Bacterial Peptides
3.4. Marine Fungus Peptides
4. Neuropeptides from Terrestrial Venomous Animal
5. Neuropeptides from Venomous Amphibian
6. Strategies for the Rational Design of Peptide as Therapeutic Leads
6.1. Peptide Design by Chemical Synthesis
6.2. Peptide Design by Molecular Biological Approach
6.3. Peptide Product by Designed Enzymatic Degradation
7. Future Perspectives of Virtual Screening on Natural Peptides
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajaretinam, R.K.; Gnana, P.V.S. Rapid neurobehavioural analysis based on the effects of an acetylcholinesterase inhibitor from Tephrosia purpurea in Zebrafish. Ann. Neurosci. 2012, 19, 8–13. [Google Scholar] [PubMed]
- Pang, Y.P.; Singh, S.K.; Gao, Y.; Lassiter, T.L.; Mishra, R.K.; Zhu, K.Y.; Brimijoin, S. Selective and irreversible inhibitors of aphid acetylcholinesterases: Steps toward human-safe insecticides. PLoS ONE 2009, 4, e4349. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, Y.; Gao, X.; Zhang, X.; Yao, J.; Pang, Y.P.; Jiang, H.; Zhu, K.Y. Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: ‘Classical’ and ‘non-classical’ functions and pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: New insights and future perspectives. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.; Braunbeck, T. Genetically engineered zebrafish liver (ZF-L) cells as an in vitro source for zebrafish acetylcholinesterase (zfAChE) for the use in AChE inhibition assays. Toxicol. In Vitro 2018, 52, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with Aβ aggregates in Alzheimer’s brain: Therapeutic relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shao, X.; Zhang, C.; Tan, Y.; Liu, Q.; Wan, X.; Zhang, Q.; Xu, S.; Jiang, X. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm. Res. 2015, 32, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer’s disease. Curr. Med. Chem. 2015, 22, 373–404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.; Zhou, Y. Quantitative multimodal multiparametric imaging in Alzheimer’s disease. Brain Inform. 2016, 3, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, C.M. Multimodal Analysis in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease: Group Differentiation, Baseline Cognition, and Predicition of Future Cognitive Decline. Ph.D. Thesis, Boston University, Boston, MA, USA, May 2013. [Google Scholar]
- Hidalgo-Muñoz, A.R.; Ramírez, J.; Górriz, J.M.; Padilla, P. Regions of interest computed by SVM wrapped method for Alzheimer’s disease examination from segmented MRI. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Hooli, B.V.; Tanzi, R.E. A current view of Alzheimer’s disease. F1000 Biol. Rep. 2009, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michele, S.; Salluzzo, M.G.; Calogero, A.E.; Raffaele, F.; Bosco, P. Association study of COX-2 (PTGS2)-765 G/C promoter polymorphism by pyrosequencing in Sicilian patients with Alzheimer’s disease. Arch. Med. Sci. 2014, 10, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Balu, D.; Melrose, J.; Chan, C. Brain region-specificity of palmitic acid-induced abnormalities associated with Alzheimer’s disease. BMC Res. Notes 2008, 1. [Google Scholar] [CrossRef] [PubMed]
- Morales-Corraliza, J.; Mazzella, M.J.; Berger, J.D.; Diaz, N.S.; Choi, J.H.; Levy, E.; Matsuoka, Y.; Planel, E.; Mathews, P.M. In vivo turnover of tau and APP metabolites in the brains of wild-type and Tg2576 mice: Greater stability of sAPP in the β-amyloid depositing mice. PLoS ONE 2009, 4, e7134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Davis, E.G.; Rebeck, G.W. Aging reduces glial uptake and promotes extracellular accumulation of Aβ from a lentiviral vector. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.-D.; Wang, Y.-D. β-Amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, R.; De Chiara, G.; Li Puma, D.D.; Ripoli, C.; Marcocci, M.E.; Garaci, E.; Palamara, A.T.; Grassi, C. HSV-1 and Alzheimer’s disease: More than a hypothesis. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xia, Y.; Pan, Y.; Chen, J. Automated identification of dementia using medical imaging: A survey from a pattern classification perspective. Brain Inform. 2016, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siuly, S.; Zhang, Y. Medical big data: Neurological diseases diagnosis through medical data analysis. Data Sci. Eng. 2016, 1, 54–64. [Google Scholar] [CrossRef]
- Dhingra, D.; Kumar, V. Memory-enhancing activity of palmatine in mice using elevated plus maze and Morris water maze. Adv. Pharmacol. Sci. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Nature: A substantial source of auspicious substances with acetylcholinesterase inhibitory action. Curr. Neuropharmacol. 2013, 11, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.L.; Jiao, S.S.; Lian, Y.; Wang, Y.J. Perspectives on the tertiary prevention strategy for Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Sorribas, A.; Howes, M.J.R. Natural products as a source of Alzheimer’s drug leads. Nat. Prod. Rep. 2011, 28, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Klafki, H.-W.; Staufenbiel, M.; Kornhuber, J.; Wiltfang, J. Therapeutic approaches to Alzheimer’s disease. Brain 2006, 129, 2840–2855. [Google Scholar] [CrossRef] [PubMed]
- Grill, J.D.; Cummings, J.L. Novel targets for Alzheimer’s disease treatment. Expert Rev. Neurother. 2010, 10, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.I.; Suresh Kumar, R.; Arumugam, N.; Basiri, A.; Kia, Y.; Ashraf Ali, M. An expedient synthesis, acetylcholinesterase inhibitory activity, and molecular modeling study of highly functionalized hexahydro-1,6-naphthyridines. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.-L.; Fu, T.-M.; Li, W.; Xu, X.-L.; Sun, H.-P. Discovery of new acetylcholinesterase inhibitors with small core structures through shape-based virtual screening. Bioorg. Med. Chem. Lett. 2015, 25, 3442–3446. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Ślusarczyk, S.; Matkowski, A.; Pérez-Garrido, A.; Girón-Rodríguez, F.; Cerón-Carrasco, J.P.; den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H.; Domaradzki, K. Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia atriplicifolia Benth. and Salvia glutinosa L. Phytochemistry 2017, 133, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Abreu, G.E.; Hernández-Aguilar, M.E.; Denes, J.M.; Hernández, L.I.G.; Rivero, M.H. Rehabilitating a brain with Alzheimer’s: A proposal. Clin. Interv. Aging 2011, 6, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S. A critical review of cholinesterase inhibitors as a treatment modality in Alzheimer’s disease. Dialogues Clin. Neurosci. 2000, 2, 111–128. [Google Scholar]
- Shah, S.; Reichman, W.E. Treatment of Alzheimer’s disease across the spectrum of severity. Clin. Interv. Aging 2006, 1, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Costantino, H.R.; Leonard, A.K.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal administration of acetylcholinesterase inhibitors. BMC Neurosci. 2008, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, J.-Y.; Zhao, Q.-H.; Liu, Y.; Gui, Y.-Z.; Liu, G.-Y.; Zhu, D.-Y.; Yu, C.; Hong, Z. Phase I study on the pharmacokinetics and tolerance of ZT-1, a prodrug of huperzine A, for the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2013, 34, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.J.; Aisen, P.S.; De Strooper, B.; Fox, N.C.; Lemere, C.A.; Ringman, J.M.; Salloway, S.; Sperling, R.A.; Windisch, M.; Xiong, C. Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Nava-Mesa, M.O.; Jiménez-Díaz, L.; Yajeya, J.; Navarro-Lopez, J.D. GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer’s disease. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, A.L.; O’Callaghan, Y.C.; O’Brien, N.M. Protein hydrolysates from agricultural crops—bioactivity and potential for functional food development. Agriculture 2013, 3, 112–130. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nut. Res. 2017, 81, 109–159. [Google Scholar]
- Min, L.J.; Kobayashi, Y.; Mogi, M.; Tsukuda, K.; Yamada, A.; Yamauchi, K.; Abe, F.; Iwanami, J.; Xiao, J.Z.; Horiuchi, M. Administration of bovine casein-derived peptide prevents cognitive decline in Alzheimer disease model mice. PLoS ONE 2017, 12, e0171515. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Furukawa, T.; Miura, S.; Akutagawa, T.; Hotta, Y.; Ishikawa, N.; Niwa, M. Antioxidant constituents in distillation residue of Awamori spirits. J. Agric. Food Chem. 2007, 55, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Hayashi, M.; Murakami, Y.; Araki, Y.; Otsuka, Y.; Kashiwagi, T.; Shimamura, T.; Ukeda, H. Development of LC-MS/MS analysis of cyclic dipeptides and its application to tea extract. Biosci. Biotechnol. Biochem. 2016, 80, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Rat, D.; Schmitt, U.; Tippmann, F.; Dewachter, I.; Theunis, C.; Wieczerzak, E.; Postina, R.; Van Leuven, F.; Fahrenholz, F.; Kojro, E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011, 25, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Blechman, J.; Levkowitz, G. Alternative splicing of the pituitary adenylate cyclase-activating polypeptide receptor PAC1: Mechanisms of fine tuning of brain activity. Front. Endocrinol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Neumann, I.D. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: Social versus non-social effects. Neuropharmacology 2012, 62, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Tang, Z.; Yin, J.; Maalouf, M.; Beach, T.G.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol. Aging 2014, 35, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [PubMed]
- Sharma, S.; Singh, R.; Rana, S. Bioactive peptides: A review. Int. J. Bioautomation 2011, 15, 223–250. [Google Scholar]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. In vitro acetylcholinesterase-inhibitory properties of enzymatic hemp seed protein hydrolysates. J. Am. Oil Chem. Soc. 2016, 93, 411–420. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, J.; Zhao, H.; Jiang, W.; Guo, X.; Zhao, M.; Sun-Waterhouse, D.; Zhao, Q.; Su, G. Antioxidant and anti-acetylcholinesterase activities of anchovy (Coilia mystus) protein hydrolysates and their memory-improving effects on scopolamine-induced amnesia mice. Int. J. Food Sci. Technol. 2017, 52, 504–510. [Google Scholar] [CrossRef]
- Zhao, T.; Su, G.; Wang, S.; Zhang, Q.; Zhang, J.; Zheng, L.; Sun, B.; Zhao, M. Neuroprotective effects of acetylcholinesterase inhibitory peptides from Anchovy (Coilia mystus) against glutamate-induced toxicity in PC12 cells. J. Agric. Food Chem. 2017, 65, 11192–11201. [Google Scholar] [CrossRef] [PubMed]
- Skiebe, P. Neuropeptides are ubiquitous chemical mediators: Using the stomatogastric nervous system as a model system. J. Exp. Biol. 2001, 204, 2035–2048. [Google Scholar] [PubMed]
- Marder, E.; Bucher, D. Central pattern generators and the control of rhythmic movements. Curr. Biol. 2001, 11, R986–R996. [Google Scholar] [CrossRef]
- Li, L.; Kelley, W.P.; Billimoria, C.P.; Christie, A.E.; Pulver, S.R.; Sweedler, J.V.; Marder, E. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J. Neurochem. 2003, 87, 642–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, B.; Priya, R.M. Bioactive Compounds from Endophytes and their Potential in Pharmaceutical Effect: A Review. Am. J. Biochem. Mol. Biol. 2011, 1, 291–309. [Google Scholar] [CrossRef]
- Bhat, Z.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Tech. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.; Araujo, M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Yen, Y.H.; Yang, J.P. Finding of polysaccharide–peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J. Food Drug Anal. 2015, 23, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.; Tsim, K.W. A Review of Dietary Ziziphus jujuba Fruit (Jujube): Developing Health Food Supplements for Brain Protection. Evid. Based Complement. Alternat. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kanbargi, K.D.; Sonawane, S.K.; Arya, S.S. Functional and antioxidant activity of Ziziphus jujube seed protein hydrolysates. J. Food Meas. Char. 2016, 10, 226–235. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Tolueinia, B.; Hashemi, A.; Ebrahimi, L.; Fesahat, F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimer’s Dis. Other Dementias 2013, 28, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Jung, I.H.; Yi, J.H.; Kim, J.H.; Park, J.H.; Lee, S.; Jung, J.W.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The Seed of Zizyphus jujuba var. spinosa Attenuates Alzheimer’s Disease-Associated Hippocampal Synaptic Deficits through BDNF/TrkB Signaling. Biol. Pharm. Bull. 2017, 40, 2096–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, L.; Cheng, Y.; Saito, M.; Yamaki, K.; Qiao, Z.; Li, L. Isoflavone content and anti-acetylcholinesterase activity in commercial Douchi (a traditional Chinese salt-fermented soybean food). Jpn. Agric. 2009, 43, 301–307. [Google Scholar] [CrossRef]
- Chen, J.; Quan, M.H.; Cheng, Y.Q.; Sun, J.; Li, L.T. Acetylcholinesterase inhibitory activity of Chinese sufu (fermented tofu) ethanol-extract. Food Chem. 2012, 134, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.-W.; You, Y.S.; Kwon, H.-S.; Yang, E.H.; Ryu, J.-S.; Kang, B.H.; Kang, J.-H. Fermented milk of Lactobacillus helveticus IDCC3801 reduces beta-amyloid and attenuates memory deficit. J. Funct. Foods 2010, 2, 143–152. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.; Mahedevan, M. Peptides derived from rice bran protect cells from obesity and Alzheimer’s disease. Int. J. Biomed. Res. 2012, 3, 131–135. [Google Scholar] [CrossRef]
- Hettiarachchy, N.S. Bioactive Pentapeptides from Rice Bran and Use Thereof. U.S. Patent 8575310B2, 4 November 2013. [Google Scholar]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2017, 33, 44–61. [Google Scholar] [CrossRef]
- Neves, J.; Campos, A.; Osório, H.; Antunes, A.; Vasconcelos, V. Conopeptides from cape verde Conus crotchii. Mar. Drugs 2013, 11, 2203–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruocco, N.; Costantini, S.; Palumbo, F.; Costantini, M. Marine sponges and bacteria as challenging sources of enzyme inhibitors for pharmacological applications. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1R–49R. [Google Scholar] [CrossRef]
- Newman, D.J.; Hill, R.T. New drugs from marine microbes: The tide is turning. J. Ind. Microbiol. Biotechnol. 2006, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Cheng, X.C.; Jensen, P.R.; Fenical, W. Scytalidamides A and B, new cytotoxic cyclic heptapeptides from a marine fungus of the genus Scytalidium. J. Org. Chem. 2003, 68, 8767–8773. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Mohamed-Ali, V.; Horakova, O.; Rossmeisl, M.; Hosseinzadeh-Attar, M.; Hensler, M.; Ruzickova, J.; Kopecky, J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006, 49, 394–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durmaz, Y. Vitamin E (α-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture 2007, 272, 717–722. [Google Scholar] [CrossRef]
- Hein, J.R.; McIntyre, B.R.; Piper, D.Z. Marine Mineral Resources of Pacific Islands-a Review of the Exclusive Economic Zones of Islands of U.S. Affiliation, Excluding the State of Hawaii; U.S. Department of the Interior, U.S. Geological Survey: Sacramento, CA, USA, 2005.
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Debashish, G.; Malay, S.; Barindra, S.; Joydeep, M. Marine enzymes. In Marine Biotechnology I; Springer: Berlin/Heidelberg, Germany, 2005; pp. 189–218. [Google Scholar]
- Thorkelsson, G.; Sigurgisladottir, S.; Geirsdottir, M.; Jóhannsson, R.; Guerard, F.; Chabeaud, A.; Bourseau, P.; Vandanjon, L.; Jaouen, P.; Chaplain-Derouiniot, M. Mild Processing Techniques and Development of Functional Marine Protein and Peptide Ingredients; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 1–59. [Google Scholar]
- Fürstner, A.; Rumbo, A. Ring-closing alkyne metathesis. Stereoselective synthesis of the cytotoxic marine alkaloid motuporamine C. J. Org. Chem. 2000, 65, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Boyd, K.G.; Mearns-Spragg, A.; Adams, D.R.; Wright, P.C.; Burgess, J.G. Two diketopiperazines and one halogenated phenol from cultures of the marine bacterium, Pseudoalteromonas luteoviolacea. Nat. Prod. Lett. 2000, 14, 435–440. [Google Scholar] [CrossRef]
- Soria-Mercado, I.E.; Prieto-Davo, A.; Jensen, P.R.; Fenical, W. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J. Nat. Prod. 2005, 68, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Deshayes, S.; Heitz, F.; Divita, G. Cell-penetrating peptides: From molecular mechanisms to therapeutics. Biol. Cell. 2008, 100, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Vink, S.; Alewood, P. Targeting voltage-gated calcium channels: Developments in peptide and small-molecule inhibitors for the treatment of neuropathic pain. Br. J. Pharmacol. 2012, 167, 970–989. [Google Scholar] [CrossRef] [PubMed]
- Newsom-Davis, J. The emerging diversity of neuromuscular junction disorders. Acta Myol. 2007, 26, 5–10. [Google Scholar] [PubMed]
- Doležal, V.; Tuček, S. Calcium channels involved in the inhibition of acetylcholine release by presynaptic muscarinic receptors in rat striatum. Br. J. Pharmacol. 1999, 127, 1627–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waqar, M.; Batool, S. In silico analysis of binding of neurotoxic venom ligands with acetylcholinesterase for therapeutic use in treatment of Alzheimer’s disease. J. Theor. Biol. 2015, 372, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sepčić, K.; Kauferstein, S.; Mebs, D.; Turk, T. Biological activities of aqueous and organic extracts from tropical marine sponges. Mar. Drugs 2010, 8, 1550–1566. [Google Scholar] [CrossRef] [PubMed]
- Beedessee, G.; Ramanjooloo, A.; Surnam-Boodhun, R.; van Soest, R.W.; Marie, D.E. Acetylcholinesterase-Inhibitory Activities of the Extracts from Sponges Collected in Mauritius Waters. Chem. Biodivers. 2013, 10, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Turk, T.; Avguštin, J.A.; Batista, U.; Strugar, G.; Kosmina, R.; Čivović, S.; Janussen, D.; Kauferstein, S.; Mebs, D.; Sepčić, K. Biological activities of ethanolic extracts from deep-sea Antarctic marine sponges. Mar. Drugs 2013, 11, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Sree, A.; Sethi, D.P.; Kumar, C.G.; Kakollu, S.; Chowdhury, L.; Dash, S.S. A marine sponge associated strain of Bacillus subtilis and other marine bacteria can produce anticholinesterase compounds. Microb. Cell. Fact. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhai, Y.; Chen, L.; Yang, Y.; Cheng, Z. Isolation and Identification of an Endophytic Bacterium with Acetylcholinesterase Inhibitory Activity Derived from Oyster and the Optimization of Fermentation Conditions. J. Qingdao Agric. Univ. 2014, 4, 1–10. [Google Scholar]
- Belofsky, G.N.; Jensen, P.R.; Fenical, W. Sansalvamide: A new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett. 1999, 40, 2913–2916. [Google Scholar] [CrossRef]
- Lee, D.-S.; Jang, J.-H.; Ko, W.; Kim, K.-S.; Sohn, J.H.; Kang, M.-S.; Ahn, J.S.; Kim, Y.-C.; Oh, H. PTP1B inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus Penicillium sp. JF-55. Mar. Drugs 2013, 11, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [PubMed]
- Wu, B.; Ohlendorf, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Acetylcholinesterase inhibitors from a marine fungus Talaromyces sp. strain LF458. Mar. Biotechnol. 2015, 17, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Eason, C.; Spurr, E. Review of the toxicity and impacts of brodifacoum on non-target wildlife in New Zealand. N. Z. J. Zool. 1995, 22, 371–379. [Google Scholar] [CrossRef]
- Hopkins, W.A. Reptile toxicology: Challenges and opportunities on the last frontier in vertebrate ecotoxicology. Environ. Toxicol. Chem. 2000, 19, 2391–2393. [Google Scholar] [CrossRef]
- Utkin, Y.N. Animal venom studies: Current benefits and future developments. World J. Biol. Chem. 2015, 6, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Porras, A.R.; Vargas, L.J.; Jimenez-Del-Rio, M.; Nuñez, V.; Velez-Pardo, C. Purification of nasulysin-1: A new toxin from Porthidium nasutum snake venom that specifically induces apoptosis in leukemia cell model through caspase-3 and apoptosis-inducing factor activation. Toxicon 2016, 120, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Inceoglu, B.; Lango, J.; Jing, J.; Chen, L.; Doymaz, F.; Pessah, I.N.; Hammock, B.D. One scorpion, two venoms: Prevenom of Parabuthus transvaalicus acts as an alternative type of venom with distinct mechanism of action. Proc. Natl. Acad. Sci. USA 2003, 100, 922–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwaltney-Brant, S.M.; Dunayer, E.; Youssef, H. Terrestrial zootoxins: In Veterinary Toxicology, 2nd ed.; Elsevier: London, UK, 2012; pp. 969–992. [Google Scholar]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowy, P.H.; Sarmiento, L.; Mitchell, H.K. Polypeptides minimine and melittin from bee venom: effects on Drosophila. Arch. Biochem. Biophys. 1971, 145, 338–343. [Google Scholar] [CrossRef]
- Nirthanan, S.; Gwee, M.C. Three-finger α-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J. Pharmacol. Sci. 2004, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ithurralde, D.; Silveira, R.; Barbeito, L.; Dajas, F. Fasciculin, a powerful anticholinesterase polypeptide from Dendroaspis angusticeps venom. Neurochem. Int. 1983, 5, 267–274. [Google Scholar] [CrossRef]
- Bowie, J.H.; Tyler, M.J. Host defense peptides from Australian amphibians: Caerulein and other neuropeptides. In Handbook of Biologically Active Peptides; Elsevier: London, UK, 2006; pp. 283–289. [Google Scholar]
- Zhang, L.; Zhang, J.; Shea, K.; Xu, L.; Tobin, G.; Knapton, A.; Sharron, S.; Rouse, R. Autophagy in pancreatic acinar cells in caerulein-treated mice: immunolocalization of related proteins and their potential as markers of pancreatitis. Toxicol. Pathol. 2014, 42, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Smyth, A.; Johnsen, A.H.; Zhou, M.; Chen, T.; Walker, B.; Shaw, C. FMRFamide-related peptides (FaRPs): Anew family of peptides from amphibian defensive skin secretions. Biochem. Biophys. Res. Commun. 2009, 383, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Siano, A.; Garibotto, F.F.; Andujar, S.A.; Baldoni, H.A.; Tonarelli, G.G.; Enriz, R.D. Molecular design and synthesis of novel peptides from amphibians skin acting as inhibitors of cholinesterase enzymes. J. Pept. Sci. 2017, 23, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; McClean, S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem. Pharmacol. 2006, 71, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.; Robichaud, J.R.; Tirrell, D.A.; Stayton, P.S.; Hoffman, A.S. The design and synthesis of polymers for eukaryotic membrane disruption. J. Control. Release 1999, 61, 137–143. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.A.; Gelmon, K.; Mayer, L.D.; Hancock, R.E.; Bally, M.B. In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des. 2000, 15, 151–160. [Google Scholar] [PubMed]
- Lewin, M.; Carlesso, N.; Tung, C.-H.; Tang, X.-W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000, 18, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Fricker, L.D.; Lim, J.; Pan, H.; Che, F.Y. Peptidomics: Identification and quantification of endogenous peptides in neuroendocrine tissues. Mass Spectrom. Rev. 2006, 25, 327–344. [Google Scholar] [CrossRef] [PubMed]
- De Spiegeleer, B.; Van Dorpe, S.; Vergote, V.; Wynendaele, E.; Pauwels, E.; Van De Wiele, C.; Garcia-Solis, P.; Solis-Sainz, J.C. In vitro metabolic stability of iodinated obestatin peptides. Peptides 2012, 33, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Chandrudu, S.; Simerska, P.; Toth, I. Chemical methods for peptide and protein production. Molecules 2013, 18, 4373–4388. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.E.; Kent, S.B. Synthesis of native proteins by chemical ligation. Annu. Rev. Biochem. 2000, 69, 923–960. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, V.R.; Ogunkoya, A.O.; Bode, J.W. Chemical protein synthesis by chemoselective α-ketoacid–hydroxylamine (KAHA) ligations with 5-oxaproline. Angew. Chem. 2012, 124, 5204–5208. [Google Scholar] [CrossRef]
- Genet, C.; Strehle, A.; Schmidt, C.; Boudjelal, G.; Lobstein, A.; Schoonjans, K.; Souchet, M.; Auwerx, J.; Saladin, R.; Wagner, A. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J. Med. Chem. 2009, 53, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Laksitorini, M.; Prasasty, V.D.; Kiptoo, P.K.; Siahaan, T.J. Pathways and progress in improving drug delivery through the intestinal mucosa and blood–brain barriers. Ther. Deliv. 2014, 5, 1143–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahan, A.; Zimmermann, E.M.; Ben-Shabat, S. Modern prodrug design for targeted oral drug delivery. Molecules 2014, 19, 16489–16505. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards improvements for penetrating the blood–brain barrier—recent progress from a material and pharmaceutical perspective. Cells 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Timmer, M.; Polanowski, A.; Lubec, G.; Trziszka, T. Manufacturing of peptides exhibiting biological activity. Amino Acids 2013, 44, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.G.; Mermod, J.-J.; Amara, S.G.; Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W.; Evans, R.M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 1983, 304, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S. Structure-function Properties of Hemp Seed Proteins and Protein-derived Acetylcholinesterase-inhibitory Peptides. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, July 2015. [Google Scholar]
- Berkov, S.; Georgieva, L.; Kondakova, V.; Atanassov, A.; Viladomat, F.; Bastida, J.; Codina, C. Plant sources of galanthamine: Phytochemical and biotechnological aspects. Biotechnol. Biotechnol. Equip. 2009, 23, 1170–1176. [Google Scholar] [CrossRef]
- Yaneva, S.; Stoykova, I.; Ilieva, L.; Vezenkov, L.; Marinkova, D.; Yotova, L.; Raykova, D.; Danalev, D. Acetylcholinesterase inhibition activity of peptide analogs of galanthamine with potential application for treatment of Alzheimer’s disease. Bulg. Chem. Commun. 2017, 49, 90–94. [Google Scholar]
- Yu, Z.; Wu, S.; Zhao, W.; Ding, L.; Fan, Y.; Shiuan, D.; Liu, J.; Chen, F. Anti-Alzheimers activity and molecular mechanism of albumin-derived peptides against AChE and BChE. Food Funct. 2018, 9, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Grisaru, D.; Deutsch, V.; Shapira, M.; Pick, M.; Sternfeld, M.; Melamed-Book, N.; Kaufer, D.; Galyam, N.; Gait, M.J.; Owen, D.; et al. ARP, a peptide derived from the stress-associated acetylcholinesterase variant, has hematopoietic growth promoting activities. Mol. Med. 2001, 7, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Yamada, M.; Hashimoto, Y.; Sato, M.; Sasabe, J.; Kita, Y.; Terashita, K.; Aiso, S.; Nishimoto, I.; Matsuoka, M. Development of a femtomolar-acting humanin derivative named colivelin by attaching activity-dependent neurotrophic factor to its N terminus: Characterization of colivelin-mediated neuroprotection against Alzheimer’s disease-relevant insults in vitro and in vivo. J. Neurosci. 2005, 25, 10252–10261. [Google Scholar] [PubMed]
- Greenfield, S.A.; Day, T.; Mann, E.O.; Bermudez, I. A novel peptide modulates α7 nicotinic receptor responses: Implications for a possible trophic-toxic mechanism within the brain. J. Neurochem. 2004, 90, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Emmett, S.R.; Greenfield, S.A. A peptide derived from the C-terminal region of acetylcholinesterase modulates extracellular concentrations of acetylcholinesterase in the rat substantia nigra. Neurosci. Lett. 2004, 358, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, S.; Morrill, P.; Tu, H.; Pottiez, G.; Badin, A.S.; Tormo-Garcia, C.; Heffner, C.; Coen, C.W.; Greenfield, S.A. (I) Pharmacological profiling of a novel modulator of the α7 nicotinic receptor: Blockade of a toxic acetylcholinesterase-derived peptide increased in Alzheimer brains. Neuropharmacology 2016, 105, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.E.; Zimmermann, M.; Greenfield, S.A. Upregulation of alpha7 Nicotinic Receptors by Acetylcholinesterase C-Terminal Peptides. PLoS ONE 2009, 4, e4846. [Google Scholar] [CrossRef] [PubMed]
- Badin, A.-S.; Eraifej, J.; Greenfield, S. High-resolution spatio-temporal bioactivity of a novel peptide revealed by optical imaging in rat orbitofrontal cortex in vitro: Possible implications for neurodegenerative diseases. Neuropharmacology 2013, 73, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L. Advances in computational approaches for drug discovery based on natural products. Rev. Latinoam. Quim. 2013, 41, 95–110. [Google Scholar]
- Medema, M.H.; Fischbach, M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.H.; Wu, J.W.; Liu, H.L.; Zhao, J.H.; Liu, K.T.; Chuang, C.K.; Lin, H.Y.; Tsai, W.B.; Ho, Y. The discovery of potential acetylcholinesterase inhibitors: A combination of pharmacophore modeling, virtual screening, and molecular docking studies. J. Biomed. Sci. 2011, 18. [Google Scholar] [CrossRef] [PubMed]
- Riswanto, F.D.O.; Hariono, M.; Yuliani, S.H.; Istyastono, E.P. Computer-aided Design of Chalcone Derivatives as Lead Compounds Targeting Acetylcholinesterase. Indonesian J. Pharm. 2017, 28, 100–111. [Google Scholar] [CrossRef]
- Mysinger, M.M.; Carchia, M.; Irwin, J.J.; Shoichet, B.K. Directory of useful decoys, enhanced (DUD-E): Better ligands and decoys for better benchmarking. J. Med. Chem. 2012, 55, 6582–6594. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Sterling, T.; Mysinger, M.M.; Bolstad, E.S.; Coleman, R.G. ZINC. A free tool to discover chemistry for biology. J. Chem. Inf. Model. 2012, 52, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.; Irwin, J.J. ZINC 15–ligand discovery for everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W. Computational methods in drug discovery. Pharmacol. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef] [PubMed]
- Ikram, N.K.K.; Durrant, J.D.; Muchtaridi, M.; Zalaludin, A.S.; Purwitasari, N.; Mohamed, N.; Rahim, A.S.A.; Lam, C.K.; Normi, Y.M.; Rahman, N.A. A virtual screening approach for identifying plants with anti H5N1 neuraminidase activity. J. Chem. Inf. Model. 2015, 55, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.A.; Moodley, N.; Steenkamp, V. Medicinal plants with cholinesterase inhibitory activity: A review. Afr. J. Biotechnol. 2010, 9, 8257–8276. [Google Scholar]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Buch, I.; Giorgino, T.; De Fabritiis, G. Complete reconstruction of an enzyme-inhibitor binding process by molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 2011, 108, 10184–10189. [Google Scholar] [CrossRef] [PubMed]

| Peptide ID | Amino Acid Sequence | IC50 (M) | Source | Parental Compound | References |
|---|---|---|---|---|---|
| I5 | Boc-VNLAG-OGal | 34.46 × 10−6 | Plant | galanthamine | [143,144] |
| KPF | KLPGF | 1.09 × 10−6 | Animal | Albumin | [145] |
| ARP | GMQGPAGSGWEEGSGSPPGVTPLFSP | n.a. | Animal | AChE-R | [146] |
| PF | EQRPR | n.a. | Plant | Protein hydrolysate | [73] |
| Colivelin | SALLRSIPAPAGASRLLLLTGEIDLP | 10−13 | Plant | ADNF C-terminally fused to AGA-(C8R)HNG17 | [147] |
| AChE-peptide | AEFHRWSSYMVHWK | 0.33 | Human | AChE | [148,149] |
| T14 | KAEFHRWSSYMVHWK | n.a. | Human | AChE | [150,151,152] |
| T15 | NQFDHYSKQDRCSDL | n.a. | Human | AChE | [150,151] |
| Compounds | Total Energy Minimum * (kcal/mol) |
|---|---|
 | −172,400.8183 |
 | −172,400.7345 |
 | −172,547.1819 |
 | −172,021.4948 |
 | −172,053.5502 |
 | −172,517.7463 |
 | −172,451.0222 |
 | −172,754.0495 |
 | −173,190.7169 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasasty, V.; Radifar, M.; Istyastono, E. Natural Peptides in Drug Discovery Targeting Acetylcholinesterase. Molecules 2018, 23, 2344. https://doi.org/10.3390/molecules23092344
Prasasty V, Radifar M, Istyastono E. Natural Peptides in Drug Discovery Targeting Acetylcholinesterase. Molecules. 2018; 23(9):2344. https://doi.org/10.3390/molecules23092344
Chicago/Turabian StylePrasasty, Vivitri, Muhammad Radifar, and Enade Istyastono. 2018. "Natural Peptides in Drug Discovery Targeting Acetylcholinesterase" Molecules 23, no. 9: 2344. https://doi.org/10.3390/molecules23092344