Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Molecules
Abstract
:1. Introduction
2. Results and Discussion
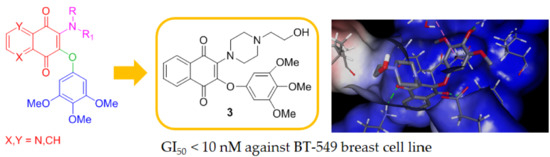
2.1. In Silico Molecular Modeling
2.2. Synthesis of Compounds 1a–c, 2a–c and 3
2.3. Biological Evaluation
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Typical Reaction Procedure for Precursors 4 and 5
3.1.3. Typical Reaction Procedure for the Synthesis of Compounds 1a–c, 2a–c and 3
3.2. Computational Analysis
3.3. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbot, V.; Sharma, P.; Dhiman, S.; Noolvi, M.N.; Patel, H.M.; Bhardwaj, V. Small hybrid heteroaromatics: Resourceful biological tools in cancer research. RSC Adv. 2017, 7, 28313–28349. [Google Scholar] [CrossRef]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Fisher, M.; Wewer, U.M.; Yoneda, A. Regulation of ROCK activity in cancer. J. Histochem. Cytochem. 2013, 61, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, S.; Li, X.; Liu, Y.; Su, J.; Chen, J. Recent advances in trimethoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2018, 151, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Li, H.P. Camptothecin and podophyllotoxin derivatives inhibitors of topoisomerase I and II—Mechanisms of Action, Pharmacokinetics and Toxicity Profile. Drug Saf. 2006, 29, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.J.; Lin, C.I.; Liou, J.P.; Kuo, C.C.; Chang, C.Y.; Chen, L.T.; Chang, J.Y. A novel synthetic microtubule inhibitor, mpt0b214 exhibits antitumor activity in human tumor cells through mitochondria-dependent intrinsic pathway. PLoS ONE 2013, 8, e58953. [Google Scholar] [CrossRef] [PubMed]
- Defant, A.; Guella, G.; Mancini, I. Synthesis and in vitro cytotoxicity evaluation of novel naphthindolizinedione derivatives. Arch. Pharm. Chem. Life Sci. 2007, 340, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Defant, A.; Guella, G.; Mancini, I. Synthesis and in-vitro cytotoxicity evaluation of novel naphtindolizinedione derivatives, part II: Improved activity for aza-analogues. Arch. Pharm. Chem. Life Sci. 2009, 342, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.Y.; Wang, P.F.; Lin, H.Y.; Tang, C.Y.; Zhu, H.L.; Yang, Y.H. Naphthoquinones: A continuing source for discovery of therapeutic antineoplastic agents. Chem. Biol. Drug Des. 2018, 91, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Liu, H.F.; Hsu, K.C.; Yang, J.M.; Chen, C.; Liu, K.K.; Hsu, T.S.; Chao, J.I. 7-Chloro-6-piperidin-1-yl-quinoline-5,8-dione (PT-262), a novel ROCK inhibitor blocks cytoskeleton function and cell migration. Biochem. Pharmacol. 2011, 81, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, druglikeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Defant, A.; Guella, G.; Mancini, I. Regioselectivity in the multi-component synthesis of indolizinoquinoline-5,12-dione derivatives. Eur. J. Org. Chem. 2006, 4201–4210. [Google Scholar] [CrossRef]
- Silver, R.F.; Holmes, H.L. Synthesis of some 1,6-naphthoquinones and reactions relating to their use in the study of bacterial growth inhibition. Can. J. Chem. 1968, 46, 1859–1864. [Google Scholar] [CrossRef]
- Egleton, J.E.; Thinnes, C.C.; Seden, P.T.; Laurieri, N.; Lee, S.P.; Hadavizadeh, K.S.; Measures, A.R.; Jones, A.M.; Thompson, S.; Varney, A.; et al. Structure–activity relationships and colorimetric properties of specific probes for the putative cancer biomarker human arylamine N-acetyltransferase 1. Bioorg. Med. Chem. 2014, 22, 3030–3054. [Google Scholar] [CrossRef] [PubMed]
- Defant, A.; Rossi, B.; Viliani, G.; Guella, G.; Mancini, I. Metal-assisted regioselectivity in nucleophilic substitutions: A study by Raman spectroscopy and density functional theory calculations. J. Raman Spectrosc. 2010, 41, 1398–1403. [Google Scholar] [CrossRef]
- NCI. Available online: https://dtp.cancer.gov/dtpstandard/cancerscreeningdata/index.jsp (accessed on 30 May 2019).
- Shaikh, I.A.; Johnson, F.; Grollman, A.P. Streptonigrin.1. Structure-activity relationship among simple bicyclic analogues. Rate dependence of DNA on quinolone reduction potential. J. Med. Chem. 1986, 29, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bosco, B.; Defant, A.; Messina, A.; Incitti, T.; Sighel, D.; Bozza, A.; Ciribilli, Y.; Inga, A.; Casarosa, S.; Mancini, I. Synthesis of 2,6-diamino-substituted purine derivatives and evaluation of cell cycle arrest in breast and colorectal cancer cells. Molecules 2018, 23, 1996. [Google Scholar] [CrossRef] [PubMed]
- Systèmes, D. BIOVIA, Discovery Studio Modeling Environment, Release 2019; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Swiss ADME. Available online: http://www.swissadme.ch/ (accessed on 28 April 2019).
- NCI (NIH). Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 30 May 2019).
Sample Availability: Samples of the compounds 1a–c, 2a–c and 3 are available from the authors. |



| Compound | Mean Growth Percent | Activity |
|---|---|---|
| 1a | 93.74 | Inactive |
| 1b | 20.92 | Active |
| 1c | 67.49 | Inactive |
| 2a | 78.44 | Inactive |
| 2b | −4.33 | Active |
| 2c | 12.79 | Active |
| 3 | 17.88 | Active |
| Cytotoxicity GI50 (μM) | |||||||
|---|---|---|---|---|---|---|---|
| 1b | 2b | 2c | 3 | Y-27632 | Podophyllotoxin | Combretastatin A-4 | |
| Cell lines | |||||||
| Leukemia | |||||||
| CCRF-CEM | 2.98 | 2.21 | 2.24 | 2.48 | 31.6 | 0.01 | 0.251 |
| HL-60(TB) | 1.76 | 0.811 | 1.43 | 1.66 | 100 | 0.01 | 0.01 |
| K-562 | 0.354 | 0.659 | 2.90 | 2.19 | 100 | - | 0.316 |
| MOLT-4 | 3.58 | 3.07 | 2.48 | 2.35 | 100 | 0.01 | 0.501 |
| RPMI-8226 | 0.486 | 1.92 | 2.23 | 2.03 | 100 | 0.01 | 0.063 |
| SR | 1.89 | 3.17 | 2.13 | 2.87 | 25.1 | 0.01 | 1.99 |
| Non-Small Cell Lung Cancer | |||||||
| A549/ATCC | 0.482 | 3.43 | 1.87 | 13.8 | 100 | 0.0126 | 0.020 |
| HOP-92 | 10.4 | 1.88 | 2.01 | 1.46 | 1.26 | 0.0316 | 0.100 |
| NCI-H226 | 0.506 | 16.67 | 2.08 | 2.92 | 100 | 0.01 | 0.251 |
| NCI-H23 | 2.11 | 2.25 | 1.76 | 2.22 | 100 | 0.01 | 0.040 |
| NCI-H322M | 6.27 | 2.65 | 7.04 | 1.23 | 100 | 0.01 | 0.063 |
| NCI-H460 | 2.77 | 3.11 | 1.65 | 3.13 | 100 | 0.01 | 0.050 |
| NCI-H522 | 1.47 | 1.46 | 1.39 | 0.571 | 100 | 0.01 | 0.032 |
| Colon Cancer | |||||||
| COLO 205 | 2.79 | 1.10 | 2.44 | 1.80 | 100 | 0.01 | 6.31 |
| HCC-2998 | 5.57 | 1.62 | 4.71 | 12.4 | 100 | 0.0126 | 0.158 |
| HCT-116 | 0.513 | 0.277 | 1.85 | 1.44 | 100 | 0.01 | 0.079 |
| HCT-15 | 0.442 | 2.07 | 2.78 | 1.56 | 100 | 0.0126 | 0.040 |
| HT29 | 3.74 | 2.36 | 3.77 | 2.27 | 100 | 0.01 | 6.31 |
| KM12 | 4.59 | 2.91 | 3.34 | 4.39 | 100 | 0.01 | 0.063 |
| SW-620 | 1.37 | 0.318 | 1.55 | 1.79 | 100 | 0.01 | 0.063 |
| CNS Cancer | |||||||
| SF-268 | 1.94 | 2.39 | 2.87 | 1.73 | 63.1 | 0.01 | 0.063 |
| SF-295 | 0.465 | 9.88 | 3.76 | 5.36 | 100 | 0.01 | 0.032 |
| SF-539 | 0.434 | 1.78 | 1.85 | 2.48 | 100 | 0.01 | 0.025 |
| SNB-19 | 0.450 | 2.00 | 2.86 | 3.41 | 100 | 0.01 | 0.025 |
| SNB-75 | 0.334 | 1.89 | 1.39 | 1.68 | 10 | 0.01 | 1.259 |
| U251 | 0.440 | 2.00 | 2.12 | 3.10 | 100 | 0.01 | 0.079 |
| Melanoma | |||||||
| LOX IMVI | 1.01 | 0.508 | 1.76 | 1.81 | 100 | 0.01 | 0.050 |
| MALME-3M | 2.73 | 0.967 | 2.23 | 3.99 | 100 | 50.1 | 0.631 |
| M14 | 0.552 | 1.84 | 3.07 | 1.96 | 100 | 0.0126 | 0.100 |
| MDA-MB-435 | 1.11 | 1.72 | 1.53 | 1.76 | 100 | 0.01 | 0.010 |
| SK-MEL-2 | 4.16 | 2.03 | 2.01 | 2.01 | 100 | 0.016 | 0.050 |
| SK-MEL-28 | 1.35 | - | 2.00 | 2.34 | 100 | 0.01 | 5.012 |
| SK-MEL-5 | 1.95 | 1.70 | 1.55 | 1.81 | 100 | 0.01 | 0.013 |
| UACC-257 | 0.846 | 1.78 | 1.56 | 3.56 | 100 | 0.01 | 0.063 |
| UACC-62 | 2.53 | 3.17 | 1.60 | 1.79 | 100 | 0.01 | 0.040 |
| Ovarian Cancer | |||||||
| OVCAR-3 | 1.77 | 0.364 | 2.02 | 1.02 | 79.4 | 0.0126 | 0.051 |
| OVCAR-4 | 1.31 | 0.937 | 1.53 | 1.63 | 100 | 0.016 | 1.995 |
| OVCAR-5 | 0.439 | 2.35 | 2.19 | 2.60 | 100 | 0.251 | 3.981 |
| OVCAR-8 | 0.463 | 0.386 | 2.13 | 2.91 | 100 | 0.01 | 0.079 |
| NCI/ADR-RES | 1.06 | 7.40 | 5.95 | 3.19 | 100 | 0.01 | 0.063 |
| SK-OV-3 | 3.28 | 11.6 | 7.49 | 5.31 | 100 | 0.01 | 0.251 |
| Renal Cancer | |||||||
| 786-0 | 2.03 | 2.46 | 2.04 | 1.93 | 100 | 0.016 | 0.631 |
| A498 | 0.640 | 2.17 | 2.09 | 2.04 | 100 | 0.01 | 0.100 |
| ACHN | 1.25 | 1.81 | 2.36 | 1.87 | 100 | 0.01 | 0.199 |
| CAKI-1 | 0.595 | 2.10 | 2.51 | 3.59 | 100 | 0.1 | 0.251 |
| RXF 393 | 0.694 | 2.00 | 2.83 | 1.55 | 100 | 0.01 | 0.398 |
| SN12C | 0.829 | 2.81 | 2.02 | 3.11 | 100 | 0.016 | 0.251 |
| TK-10 | 3.27 | 3.63 | 3.91 | 4.97 | 10 | 0.0316 | 3.162 |
| UO-31 | 1.18 | 1.80 | 1.29 | 1.61 | 100 | 0.016 | 1.000 |
| Prostate Cancer | |||||||
| PC-3 | 0.914 | 2.43 | 2.72 | 3.19 | 100 | 0.01 | 0.010 |
| DU-145 | 2.60 | 3.43 | 4.19 | - | 100 | 0.01 | 0.013 |
| Breast Cancer | |||||||
| MCF7 | 0.509 | 0.304 | 1.20 | 1.15 | 100 | 0.01 | 0.010 |
| MDA-MB-231/ATCC | 4.14 | 2.55 | 1.81 | 2.00 | 100 | 0.01 | 0.016 |
| HS 578T | 0.415 | 3.06 | 2.84 | 2.69 | 100 | 0.01 | 0.010 |
| BT-549 | 0.819 | 2.68 | 4.96 | <0.01 | 100 | 0.01 | 0.020 |
| T-47D | 2.53 | 1.67 | 1.98 | 0.510 | 100 | 79.4 | 50.12 |
| MDA-MB-468 | 1.77 | 0.133 | 0.285 | 1.37 | 100 | 0.01 | 0.079 |
| MGMa) | 1.29 | 1.82 | 2.24 | 2.24 | 91.6 | 1.52 | 1.52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Defant, A.; Mancini, I. Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Molecules. Molecules 2019, 24, 2224. https://doi.org/10.3390/molecules24122224
Defant A, Mancini I. Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Molecules. Molecules. 2019; 24(12):2224. https://doi.org/10.3390/molecules24122224
Chicago/Turabian StyleDefant, Andrea, and Ines Mancini. 2019. "Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Molecules" Molecules 24, no. 12: 2224. https://doi.org/10.3390/molecules24122224