Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation
Abstract
:1. Introduction
2. Results
2.1. GAT211 and GAT229 Potentiated the Anti-Nociceptive Effects of Δ8-THC, Whereas GAT228 Directly Reduced Corneal Pain
2.2. GAT229 and GAT228 Reduce Corneal Pain via Activation of CB1
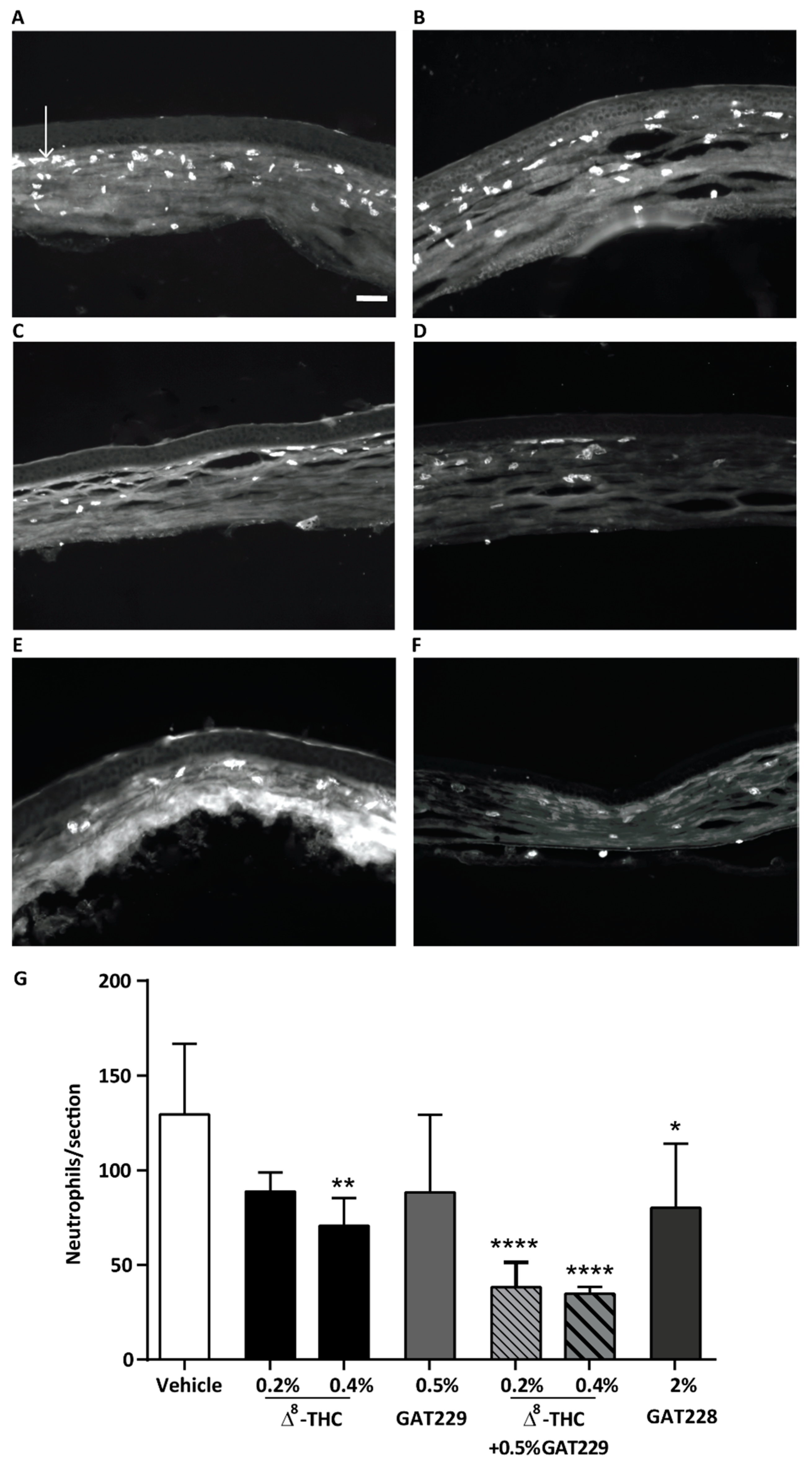
2.3. GAT229 in Combination with Δ8-THC, and GAT228 Alone, Reduced Neutrophil Infiltration to the Cornea
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Induction of Corneal Injury
4.3. Assessment of Behavioral Pain Response
4.4. Neutrophil Migration
4.5. Pharmacological Treatments
4.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belmonte, C.; Aracil, A.; Acosta, M.C.; Luna, C.; Gallar, J. Nerves and sensations from the eye surface. Ocul. Surf. 2004, 2, 248–253. [Google Scholar] [CrossRef]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- LaFreniere, J.D.; Kelly, M.E. Potential for endocannabinoid system modulation in ocular pain and inflammation: Filling the gaps in current pharmacological options. Neuronal Signal. 2018, 2, NS20170144. [Google Scholar] [CrossRef] [Green Version]
- Assam, J.H.; Bernhisel, A.; Lin, A. Intraoperative and postoperative pain in cataract surgery. Surv. Ophthalmol. 2018, 63, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; House, R.J.; Feldman, B.H. Corneal Abrasions and Corneal Foreign Bodies. Prim. Care Clin. Off. Pr. 2015, 42, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Millodot, M. Effect of Long-term Wear of Hard Contact Lenses on Corneal Sensitivity. Arch. Ophthalmol. 1978, 96, 1225–1227. [Google Scholar] [CrossRef]
- Tabatabaei, S.A.; Soleimani, M.; Johari, M. Corneal ring infiltration in contact lens wearers. Oman J. Ophthalmol. 2017, 10, 106–108. [Google Scholar]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Merayo-Lloves, J.; Gallar, J. What Causes Eye Pain? Curr. Ophthalmol. Rep. 2015, 3, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Akpek, E.K.; Gottsch, J.D. Immune defense at the ocular surface. Eye 2003, 17, 949–956. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galor, A.; Moein, H.-R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Hamrah, P. Understanding Neuropathic Corneal Pain—Gaps and Current Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, P.; Borsook, D. Ocular neuropathic pain. Br. J. Ophthalmol. 2016, 100, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef] [PubMed]
- Namavari, A.; Chaudhary, S.; Chang, J.-H.; Yco, L.; Sonawane, S.; Khanolkar, V.; Yue, B.Y.; Sarkar, J.; Jain, S. Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves. Investig. Opthalmology Vis. Sci. 2012, 53, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, D.S. Diagnosis and Treatment of Ocular Pain: The Ophthalmologist’s Perspective. Curr. Ophthalmol. Rep. 2017, 5, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-J.; Chen, W.-W.; Zhang, X. Endocannabinoid system: Role in depression, reward and pain control (Review). Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Oddi, S.; Piccoli, A.; Fazio, D.; Maccarrone, M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016, 111, 721–730. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.; Wang, Z.; Varadaraj, K.; Kumari, S.S.; Mergler, S.; Okada, Y.; Saika, S.; Kingsley, P.J.; Marnett, L.J.; et al. Cannabinoid receptor 1 suppresses transient receptor potential vanilloid 1-induced inflammatory responses to corneal injury. Cell Signal 2013, 25, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Breivogel, C.S.; Childers, S.R.; Deadwyler, S.A.; Hampson, R.E.; Porrino, L.J. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 2004, 47, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. Cannabinoid Receptor Signaling. In Handbook of Experimental Pharmacology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; Volume 168, pp. 53–79. [Google Scholar]
- Alger, B.E.; Kim, J. Supply and demand for endocannabinoids. Trends Neurosci. 2011, 34, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V. Endocannabinoid signaling in the brain: Biosynthetic mechanisms in the limelight. Nat. Neurosci. 2011, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Ambwani, S.R.; Singh, S. Endocannabinoid System: A multi-facet therapeutic target. Curr. Clin. Pharmacol. 2016, 11, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R.G. Endocannabinoids and Their Pharmacological Actions. In Handbook of Experimental Pharmacology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; Volume 231, pp. 1–37. [Google Scholar]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.M.; Toguri, J.T.; Caldwell, M.D.; Kelly, M.E.M. The Cannabinoids Delta(8)THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation. Cannabis. Cannabinoid. Res. 2018, 3, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Burston, J.J.; Wiley, J.L.; Craig, A.A.; Selley, D.E.; Sim-Selley, L.J. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br. J. Pharmacol. 2010, 161, 103–112. [Google Scholar] [CrossRef] [Green Version]
- González, S.; Cebeira, M.; Fernandezruiz, J. Cannabinoid tolerance and dependence: A review of studies in laboratory animals. Pharmacol. Biochem. Behav. 2005, 81, 300–318. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Martin, B.R. Cannabinoid tolerance and dependence. In Handbook of Experimental Pharmacology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; Volume 168, pp. 691–717. [Google Scholar]
- Ross, R.A. Allosterism and cannabinoid CB1 receptors: The shape of things to come. Trends Pharmacol. Sci. 2007, 28, 567–572. [Google Scholar] [CrossRef]
- Khurana, L.; Mackie, K.; Piomelli, D.; Kendall, D.A. Modulation of CB1 cannabinoid receptor by allosteric ligands: Pharmacology and therapeutic opportunities. Neuropharmacology 2017, 124, 3–12. [Google Scholar] [CrossRef] [Green Version]
- LaPrairie, R.B.; Kulkarni, P.M.; Deschamps, J.R.; Kelly, M.E.M.; Janero, D.R.; Cascio, M.G.; Stevenson, L.A.; Pertwee, R.G.; Kenakin, T.P.; Denovan-Wright, E.M.; et al. Enantiospecific Allosteric Modulation of Cannabinoid 1 Receptor. ACS Chem. Neurosci. 2017, 8, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Slivicki, R.A.; Xu, Z.; Kulkarni, P.M.; Pertwee, R.G.; Mackie, K.; Thakur, G.A.; Hohmann, A.G. Positive Allosteric Modulation of Cannabinoid Receptor Type 1 Suppresses Pathological Pain Without Producing Tolerance or Dependence. Biol. Psychiatry 2018, 84, 722–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatowska-Jankowska, B.M.; Baillie, G.L.; Kinsey, S.; Crowe, M.; Ghosh, S.; Owens, R.A.; Damaj, I.M.; Poklis, J.; Wiley, J.L.; Zanda, M.; et al. A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology 2015, 40, 2948–2959. [Google Scholar] [CrossRef] [Green Version]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Investig. 2005, 115, 2393–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allchorne, A.J.; Gooding, H.L.; Mitchell, R.; Fleetwood-Walker, S.M. A novel model of combined neuropathic and inflammatory pain displaying long-lasting allodynia and spontaneous pain-like behaviour. Neurosci. Res. 2012, 74, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; McDowell, T.; Wang, P.; Alvarez, R.; Gomez, T.; Bjorling, D.E. Activation of CB1 inhibits NGF-induced sensitization of TRPV1 in adult mouse afferent neurons. Neuroscience 2014, 277, 679–689. [Google Scholar] [CrossRef] [Green Version]
- McDowell, T.S.; Wang, Z.-Y.; Singh, R.; Bjorling, D. CB1 cannabinoid receptor agonist prevents NGF-induced sensitization of TRPV1 in sensory neurons. Neurosci. Lett. 2013, 551, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Walczak, J.-S.; Cervero, F. Local activation of cannabinoid CB1 receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol. Pain 2011, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Murataeva, N.; Li, S.; Oehler, O.; Miller, S.; Dhopeshwarkar, A.; Hu, S.S.-J.; Bonanno, J.A.; Bradshaw, H.; Mackie, K.; McHugh, U.; et al. Cannabinoid-Induced Chemotaxis in Bovine Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2015, 56, 3304–3313. [Google Scholar] [CrossRef]
- Janero, D.R.; Thakur, G.A. Leveraging allostery to improve G protein-coupled receptor (GPCR)-directed therapeutics: Cannabinoid receptor 1 as discovery target. Expert Opin. Drug Discov. 2016, 11, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Alaverdashvili, M.; LaPrairie, R.B. The future of type 1 cannabinoid receptor allosteric ligands. Drug Metab. Rev. 2018, 50, 14–25. [Google Scholar] [CrossRef] [PubMed]
- LaPrairie, R.B.; Kulkarni, A.R.; Kulkarni, P.M.; Hurst, W.P.; Lynch, D.L.; Reggio, P.H.; Janero, D.R.; Pertwee, R.G.; Stevenson, L.A.; Kelly, M.E.M.; et al. Mapping Cannabinoid 1 Receptor Allosteric Site(s): Critical Molecular Determinant and Signaling Profile of GAT100, a Novel, Potent, and Irreversibly Binding Probe. ACS Chem. Neurosci. 2016, 7, 776–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenakin, T.P. Biased signalling and allosteric machines: New vistas and challenges for drug discovery. Br. J. Pharmacol. 2012, 165, 1659–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, M.R.; Finlay, D.B.; Macdonald, C.E.; Cawston, E.E.; Grimsey, N.L.; Glass, M. Real-Time Measurement of Cannabinoid Receptor-Mediated cAMP Signaling. Methods Enzymol. 2017, 593, 43–59. [Google Scholar] [PubMed]
- Cawston, E.E.; Redmond, W.J.; Breen, C.M.; Grimsey, N.L.; Connor, M.; Glass, M. Real-time characterization of cannabinoid receptor 1 (CB1) allosteric modulators reveals novel mechanism of action. Br. J. Pharmacol. 2013, 170, 893–907. [Google Scholar] [CrossRef] [Green Version]
- Cairns, E.A.; Szczesniak, A.-M.; Straiker, A.J.; Kulkarni, P.M.; Pertwee, R.G.; Thakur, G.A.; Baldridge, W.H.; Kelly, M.E. The In Vivo Effects of the CB1-Positive Allosteric Modulator GAT229 on Intraocular Pressure in Ocular Normotensive and Hypertensive Mice. J. Ocul. Pharmacol. Ther. 2017, 33, 582–590. [Google Scholar] [CrossRef]
- Petrosino, S.; Palazzo, E.; De Novellis, V.; Bisogno, T.; Rossi, F.; Maione, S.; Di Marzo, V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology 2007, 52, 415–422. [Google Scholar] [CrossRef]
- Mitrirattanakul, S.; Ramakul, N.; Guerrero, A.V.; Matsuka, Y.; Ono, T.; Iwase, H.; Mackie, K.; Faull, K.F.; Spigelman, I. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain 2006, 126, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Oltmanns, M.H.; Samudre, S.S.; Castillo, I.G.; Hosseini, A.; Lichtman, A.H.; Allen, R.C.; Lattanzio, F.A.; Williams, P.B. Topical WIN55212-2 alleviates intraocular hypertension in rats through a CB1 receptor mediated mechanism of action. J. Ocul. Pharmacol. Ther. 2008, 24, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Changeux, J.-P.; Christopoulos, A. Allosteric Modulation as a Unifying Mechanism for Receptor Function and Regulation. Cell 2016, 166, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Li, J.X.; Thomas, B.F.; Wiley, J.L.; Kenakin, T.P.; Zhang, Y. Allosteric Modulation: An Alternate Approach Targeting the Cannabinoid CB1 Receptor. Med. Res. Rev. 2017, 37, 441–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, N.; Hucke, O.; Kramer, G.; Schmidt, E.; Montel, F.; Lipinski, R.; Ferger, B.; Clark, T.; Hildebrand, P.W.; Tautermann, C.S. Multiple Binding Sites Contribute to the Mechanism of Mixed Agonistic and Positive Allosteric Modulators of the Cannabinoid CB1 Receptor. Angew. Chem. Int. Ed. Engl. 2018, 57, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Wenk, H.; Honda, C.; Wenk, N.H.; Honda, N.C. Silver nitrate cauterization: Characterization of a new model of corneal inflammation and hyperalgesia in rat. Pain 2003, 105, 393–401. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds GAT211, GAT228, and GAT229 are available from the G.A.T. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, D.; Cairns, E.A.; Szczesniak, A.-M.; Kulkarni, P.M.; Straiker, A.J.; Thakur, G.A.; Kelly, M.E.M. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules 2020, 25, 417. https://doi.org/10.3390/molecules25020417
Thapa D, Cairns EA, Szczesniak A-M, Kulkarni PM, Straiker AJ, Thakur GA, Kelly MEM. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules. 2020; 25(2):417. https://doi.org/10.3390/molecules25020417
Chicago/Turabian StyleThapa, Dinesh, Elizabeth A. Cairns, Anna-Maria Szczesniak, Pushkar M. Kulkarni, Alex J. Straiker, Ganesh A. Thakur, and Melanie E. M. Kelly. 2020. "Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation" Molecules 25, no. 2: 417. https://doi.org/10.3390/molecules25020417





