Evaluation of Effect of Microwave Irradiation on Syntheses and Reactions of Some New 3-Acyl-methylchromones
Abstract
:Introduction
Results and Discussions
| Comp | Formula | M.P., °C | Calc. / Found | ν(C=O)c | ν(C=O)c | ν(C=N)c | ν(OH)c | |||
| Yield, % | M.W. | Solvent | %C | %H | %N | %Cl | pyrone | acetyl | ||
| 2a | C12H10O3 | 86-87 | 71.28 | 4.98 | 1637 | 1687 | ||||
| 72 | 202.21 | P.Etherb | 71.56 | 5.07 | ||||||
| 2b | C12H12O3 | 116-118 | 72.21 | 5.59 | 1639 | 1691 | ||||
| 85 | 216.24 | Cyclohexa | 72.45 | 5.64 | ||||||
| 2c | C12H9ClO3 | 129-131 | 60.90 | 3.83 | 14.98 | 1639 | 1691 | |||
| 82 | 236.65 | Cyclohexa | 60.77 | 3.84 | 14.98 | |||||
| 2d | C12H9BrO3 | 124-125 | 51.27 | 3.23 | 28.42 | 1640 | 1692 | |||
| 82 | 281.11 | Cyclohexa | 51.31 | 3.17 | 28.63 | |||||
| 2e | C12H8Cl2O3 | 132-134 | 53.17 | 2.97 | 26.15 | 1643 | 1680 | |||
| 98 | 271.10 | Cyclohexa | 53.40 | 3.01 | 26.18 | |||||
| 2f | C13H11ClO3 | 152-153 | 62.29 | 4.42 | 14.14 | 1637 | 1687 | |||
| 91 | 250.68 | Cyclohexa | 62.56 | 4.45 | 14.29 | |||||
| 2g | C14H14O3 | 112-114 | 73.03 | 6.13 | 1636 | 1677 | ||||
| 84 | 230.26 | Cyclohexa | 73.31 | 6.14 | ||||||
| 2h | C16H12O3 | 154-156 | 76.18 | 4.79 | 1637 | 1685 | ||||
| 91 | 252.27 | Cyclohexa | 76.22 | 4.81 | ||||||
| 2i | C16H12O3 | 136-138 | 76.18 | 4.79 | 1648 | 1699 | ||||
| 95 | 252.27 | Cyclohexa | 76.24 | 4.79 | ||||||
| 5a | C17H12O3 | 113.5-115 | 77.21 | 4.54 | ||||||
| 73 | 264.2 | Cyclohexa | 77.38 | 4.41 | ||||||
| 5b | C18H14O3 | 90-93 | 77.64 | 5.03 | ||||||
| 26 | 278.22 | Cyclohexa | 77.52 | 5.11 | ||||||
| 5c | C17H11BrO3 | 141-143 | 59.48 | 3.21 | ||||||
| 74 | 343.2 | Cyclohexa | 59.73 | 3.29 | ||||||
| 5d | C21H16O3 | 210-212 | 80.20 | 4.45 | ||||||
| 72 | 314.2 | Dioxane | 80.09 | 4.32 | ||||||
| 7a | C19H16Cl2O5 | 119-121 | 57.74 | 4.08 | 17.94 | |||||
| 21 | 395.24 | Cyclohexa | 57.32 | 4.02 | 17.77 | |||||
| 7b | C19H15Cl3O5 | 94-95 | 53.11 | 3.52 | 24.75 | |||||
| 18 | 429.68 | Cyclohexa | 53.50 | 3.45 | 24.50 | |||||
| 7c | C19H16Cl2O5 | 104-105 | 57.74 | 4.08 | 17.94 | |||||
| 25 | 395.24 | Cyclohexa | 52.85 | 4.19 | 17.75 | |||||
| 7f | C24H17NO8 | 112-114 | 64.43 | 3.83 | 3.13 | |||||
| 18 | 447.40 | Toluene | 64.28 | 3.72 | 3.15 | |||||
| 8a | C19H14Cl2O4 | 150-151 | 60.80 | 3.74 | 18.80 | |||||
| 377.22 | Ethanole | 59.96 | 3.69 | 18.82 | ||||||
| 8b | C19H13Cl3O4 | 187-189 | 55.44 | 3.18 | 25.84 | |||||
| 411.67 | Ethanole | 55.25 | 3.18 | 25.95 | ||||||
| 8c | C19H14Cl2O4 | 145-148 | 60.50 | 3.74 | 18.89 | |||||
| 377.20 | Ethanole | 60.44 | 3.52 | 18.01 | ||||||
| 8d | C20H16Cl2O4 | 127-129 | 61.40 | 4.12 | 18.12 | |||||
| 391.25 | Ethanole | 61.28 | 4.05 | 18.25 | ||||||
| 8e | C20H17ClO4 | 153-156 | 67.33 | 4.80 | 9.94 | |||||
| 356.81 | Ethanole | 67.45 | 4.92 | 10.23 | ||||||
| 8f | C21H15NO5 | 241-244 | ||||||||
| 9a | C13H12ClNO3 | 114-115 | 58.77 | 4.55 | 5.27 | 13.34 | 1683 | 1612 | 3100 | |
| 57 | 265.70 | Cyclohexa | 58.46 | 4.55 | 5.06 | 13.58 | (br) | |||
| 9b | C14H15NO3 | 119-121 | 68.56 | 6.16 | 5.71 | 1680 | 1613 | 3100 | ||
| 62 | 245.28 | Cyclohexa | 68.55 | 6.19 | 5.52 | (br) | ||||
| 9c | C17H13NO3 | 73-75 | 73.12 | 4.66 | 5.02 | |||||
| 48 | 279.2 | Cyclohexa | 73.17 | 4.82 | 4.88 | |||||
| 9d | C18H15NO3 | 90-92 | 73.72 | 5.12 | 4.78 | |||||
| 50 | 293.2 | Cyclohexa | 73.72 | 5.21 | 4.79 | |||||
| 9e | C17H12BrNO3 | 121-123 | 56.98 | 3.35 | 3.91 | |||||
| 52 | 358.2 | 57.12 | 3.38 | 3.94 | ||||||
| 10a | C13H12ClNO3 | 150-151 | 58.77 | 4.55 | 5.27 | 13.34 | 1681 | 1620 | 3120 | |
| 20 | 265.70 | Benzene | 58.35 | 4.60 | 5.02 | 13.61 | ||||
| 10b | C14H15NO3 | 142-144 | 68.56 | 6.16 | 5.71 | 1675 | 1620 | 3127 | ||
| 28 | 245.28 | Benzene | 68.61 | 6.16 | 5.74 | |||||
| 10c | C14H15NO3 | 212-214 | 73.12 | 4.66 | 5.02 | |||||
| 33 | 279.2 | Toluene | 73.15 | 4.70 | 5.06 | |||||
| 10d | C18H15NO3 | 182-187 | 73.72 | 5.12 | 4.78 | |||||
| 35 | 293.2 | Toluene | 73.64 | 5.20 | 4.72 | |||||
| 10e | C17H12BrNO3 | 221-223 | 56.98 | 3.35 | 3.91 | |||||
| 24 | 358.2 | Toluene | 57.04 | 3.34 | 3.86 | |||||
| 11a | C12H22O5 | 174-175 | 73.74 | 3.94 | ||||||
| 358.35 | Ethanol | 73.64 | 3.70 | |||||||
| 11b | C23H16O5 | 289-291 | 74.19 | 4.33 | ||||||
| 372.38 | Ethanol | 73.99 | 4.23 | |||||||
| 11c | C22H13ClO5 | 270-273 | 67.27 | 3.34 | 9.03 | |||||
| 392.80 | Ethanol | 67.17 | 3.10 | 8.93 | ||||||
| 11d | C23H15ClO5 | 264-266 | 67.91 | 3.72 | 8.71 | |||||
| 406.85 | Ethanol | 67.70 | 3.52 | 8.50 | ||||||
| 11e | C24H18O5 | 224-226 | 74.60 | 4.70 | ||||||
| 386.40 | Ethanol | 74.40 | 4.65 | |||||||
| 11f | C22H12Cl2O5 | 300-301 | 61.85 | 2.83 | 16.60 | |||||
| 427.20 | DM-Etc | 61.59 | 2.73 | 16.73 | ||||||
| 11g | C22H13Cl2O5 | 265-267 | 67.27 | 3.34 | 9.03 | |||||
| 392.8 | Ethanol | 67.10 | 3.20 | 9.09 | ||||||
Experimental Section
General
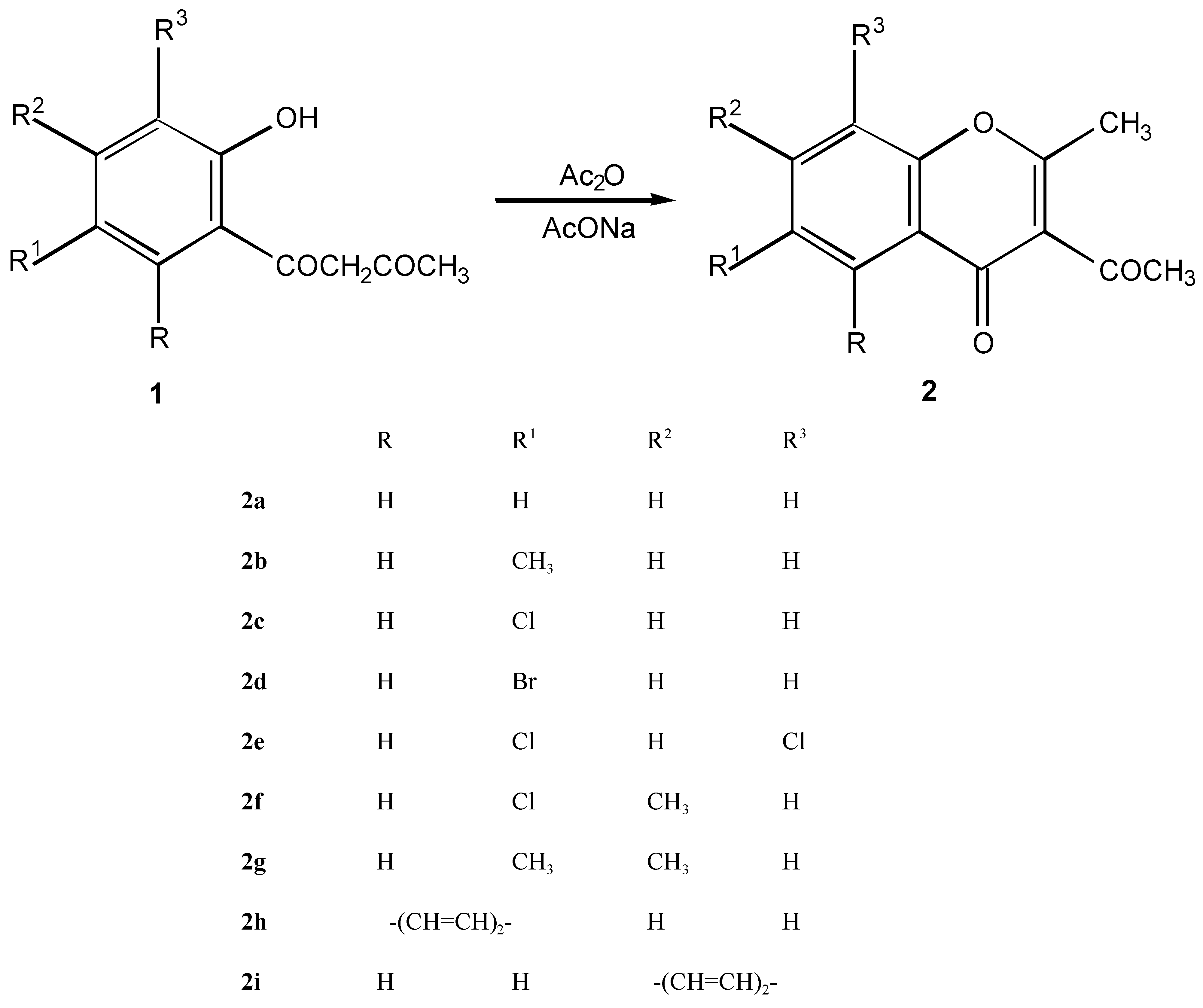
3-Acetyl-2-methylchromone derivatives 2a - 2i
Method A (classic)
Method B ( microwave irradiation)
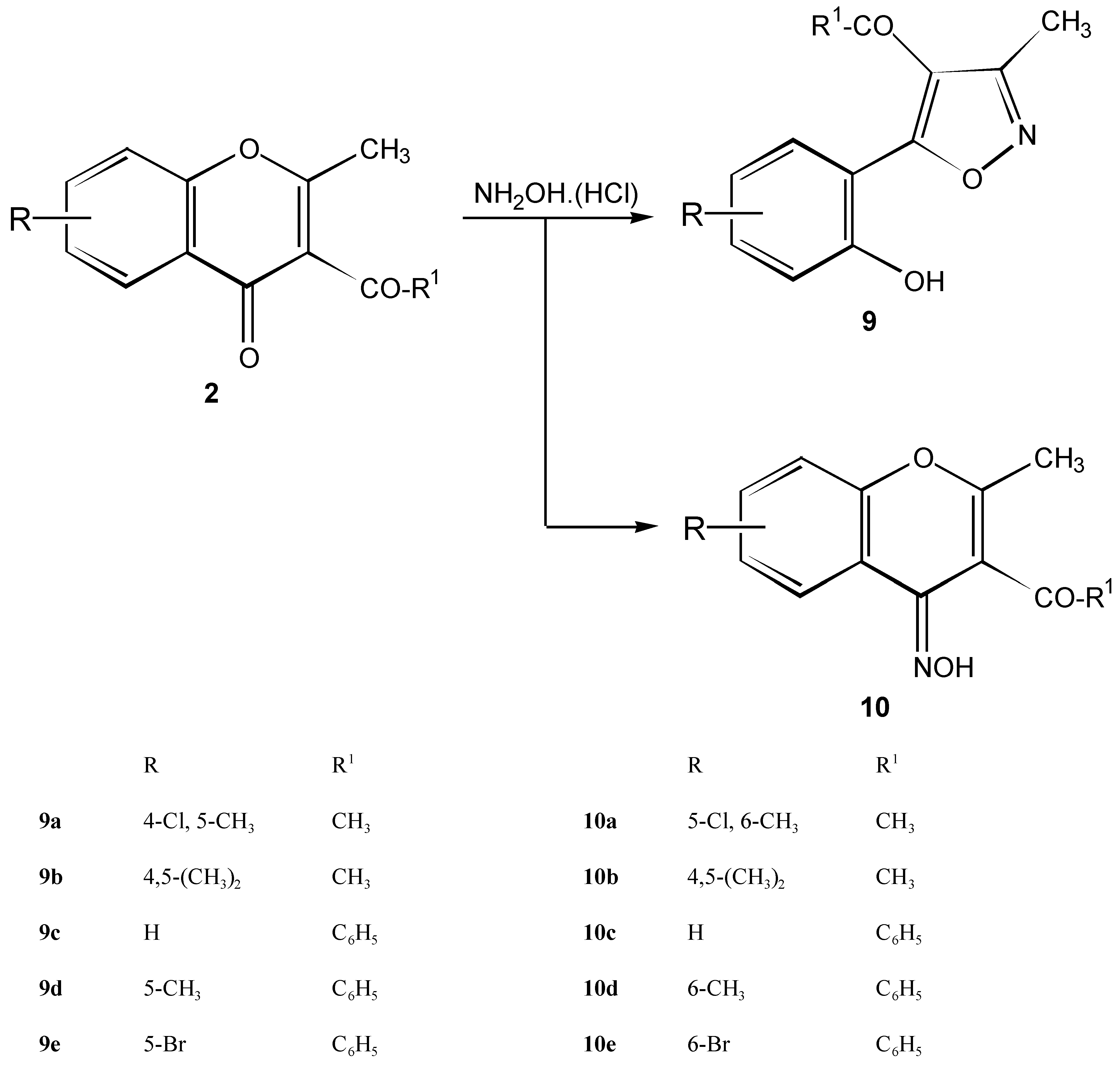
4-Acetyl-5-(2-hydroxyaryl)-3-methylisoxazoles 9a - 9e and 4-(3-acetyl-2, 7-dimethylchromone)-oximes 10a - 10e
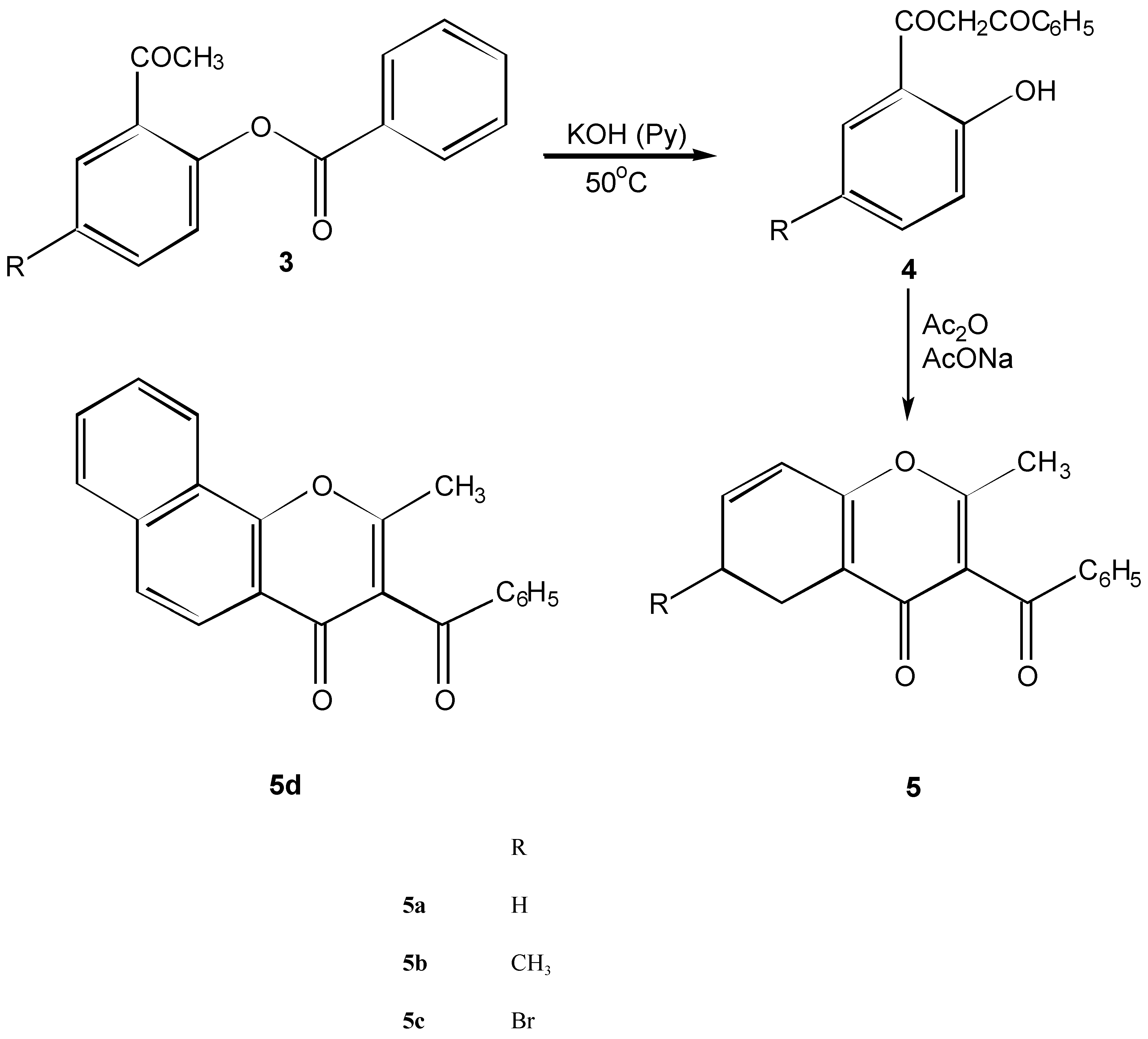
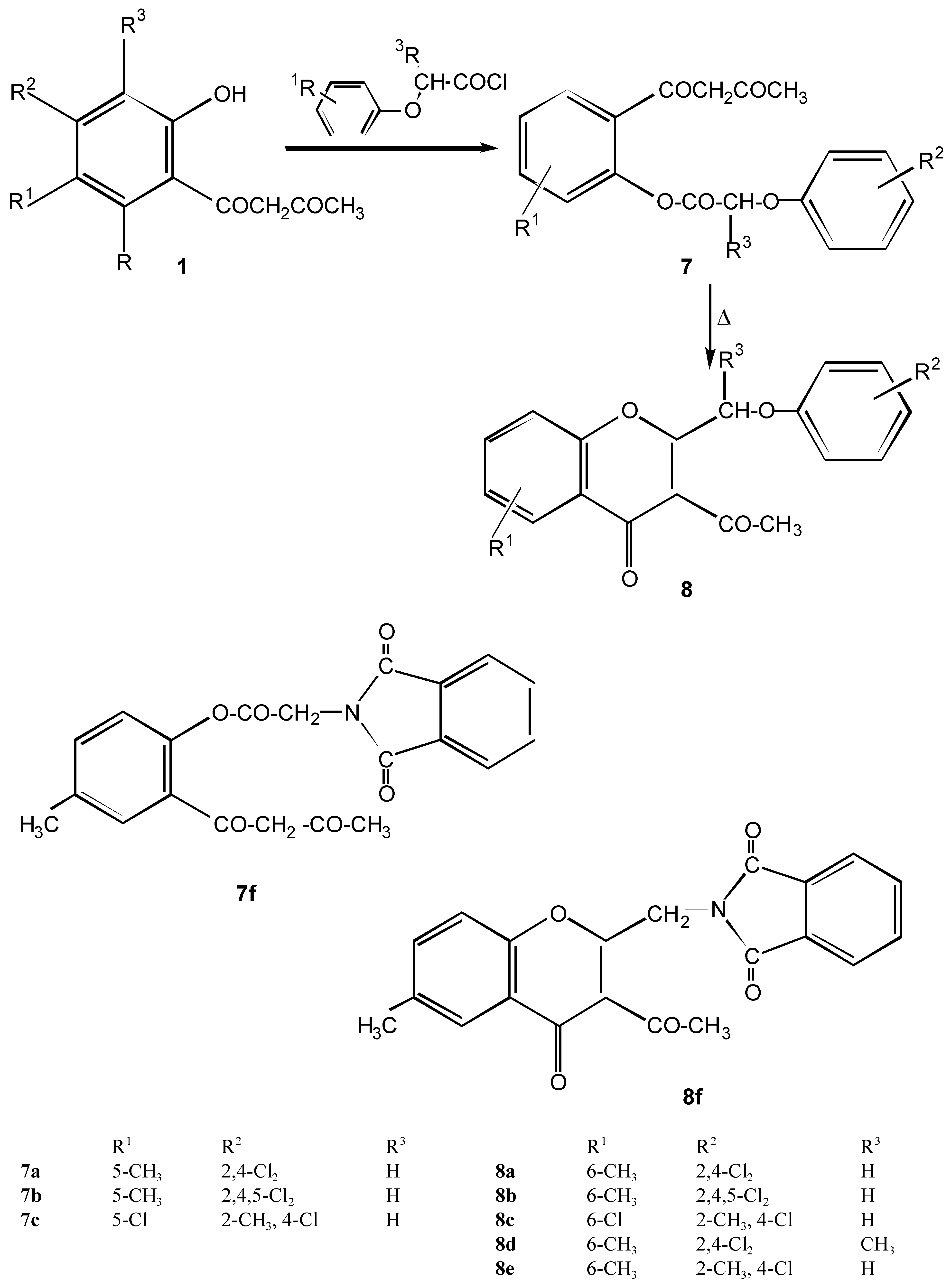
2-Aryloxymethyl-3-acetylchromone derivatives 8a - 8e and intermediates 7a - 7c
Method A
Method B
Method C
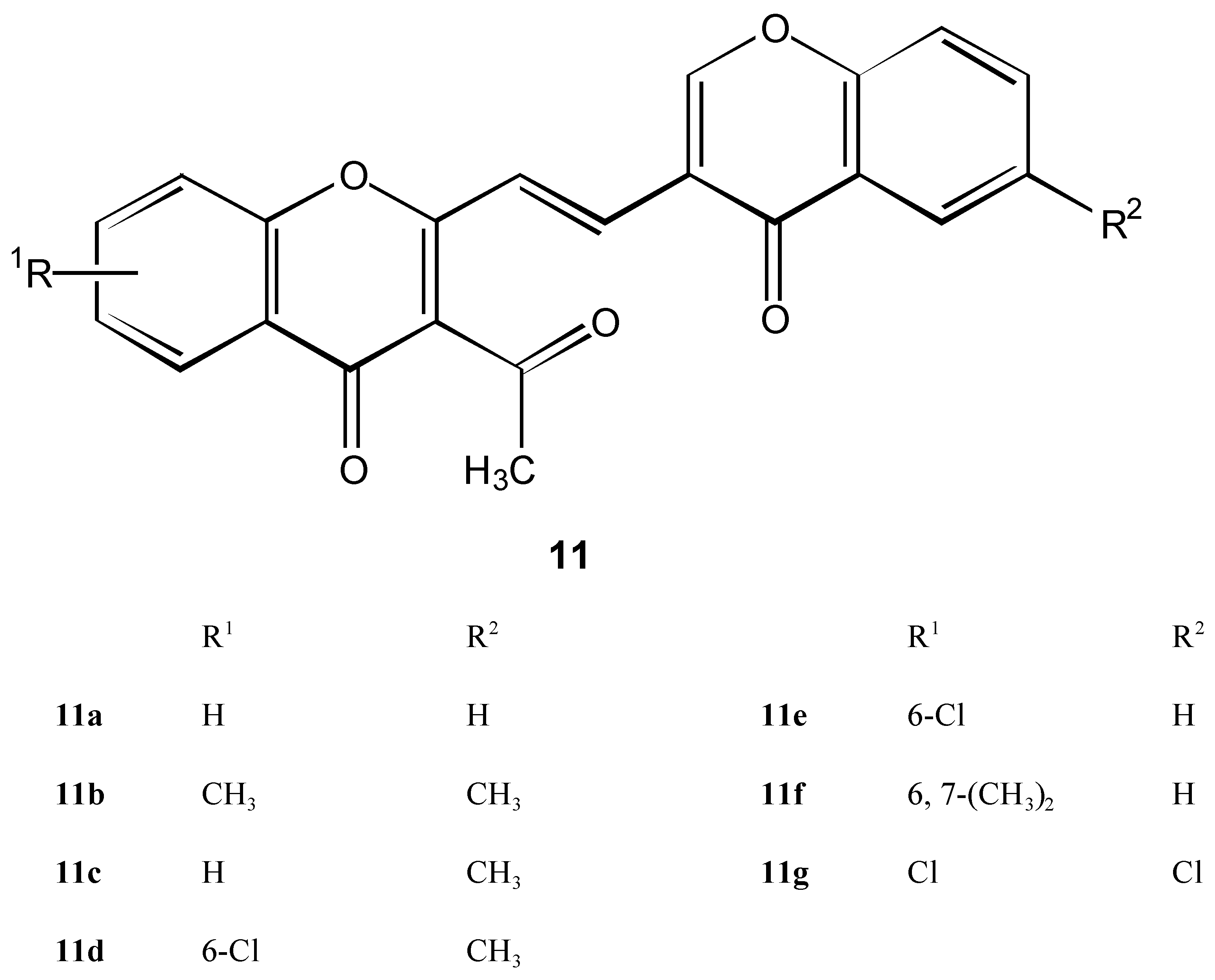
Condensation products 11 of 2 with 3-formylchromones 6
Method A ( classic)
Method B
Acknowledgement
References
- Stankovicova, H.; Fabian, W. M. F.; Lácová, M. Molecules 1996, 1, 223–235.
- Gasparova, R.; Lácová, M. Collect. Czech. Chem. Commun. 1995, 60, 1178–1185.
- Lácová, M.; Stankovicova, H.; Odlerová, Z. Il Pharmaco 1995, 50, 885.
- El-Shaaer, H. M.; Perjéssy, A.; Zahradník, P.; Lácová, M.; Matulová, M. Monatsh. Chem. 1993, 124, 539.
- Kostka, K. Roczniky Chem. 1996, 40, 1683.
- Ghosh, C. K. J. Heterocyclic Chem. 1983, 20, 1437.
- Masayuki, K.; Kunio, H. Jpn. Kokai: Tokkyo Koho. JP 62 77, 377, 09 Apr 1987. Appl. 30 Sep 1985. [Google Scholar]
- Desai, R. D.; Vakil, V. M. Proc. Indian Acad. Sci. 1940, 13A, 357.
- Shah, M. V.; Sethna, S. J. Chem. Soc. 1961, 2663.
- Wittig, G.; Bangert, F. Ber. 1925, 58, 2627.
- Ghosh, C. K.; Pal, C.; Bhattacharyya, A. Indian J. Chem. 1985, 24B, 914.
- Sample Availability: Available from the MDPI.
| Compound | 1H-NMR spectruma (solvent CDCl3 or DMSOx) δ (ppm) |
| 2a | 8.14(1H, dd, 3J=8.4 and 1.6, H-5), 7.64(1H, ddd, 3J=7.1, 8.2 and 1.6, H-7), 7.39(1H, dd, 4J=8.2 and 1.1, H-8), 7.37(1H, ddd, J=8.4, 7.1 and 1.1, H-6), 2.63(3H, s, CH3 acetyl), and 2.52(3H, s, C2-CH3). |
| 2b | 7.99(1H, d, 4J=2.3, H-5), 7.50(1H, dd, 4J=8.7 and 4J=2.3, H-7), 7.34(1H, d, 3J=8.7, H-8), 2.67(3H, s, CH3 acetyl), 2.55(3H, s, C2-CH3), and 2.45(3H, s, C6-CH3). |
| 2c | 8.14(1H, d, 4J=2.6, H-5), 7.60(1H, dd, 3J=8.8 and 4J=2.6, H-7), 7.38(1H, d, 3J=8.8, H-8), 2.64(3H, s, CH3 acetyl), and 2.54(3H, s, C2-CH3). |
| 2d | 8.30(1H, d, 4J=2.4, H-5), 7.76(1H, dd, 3J=8.8 and 4J=2.4, H-7), 7.33(1H, d, 3J=8.8, H-8), 2.64(3H, s, CH3 acetyl), and 2.53(3H, s, C2-CH3). |
| 2e | 8.04(1H, d, 4J=2.2, H-5), 7.70(1H, d, 4J=2.2, H-7), 2.62(3H, s, CH3 acetyl), and 2.60(3H, s, C2-CH3). |
| 2f | 8.12(1H, s, H-5), 7.33(1H, s, H-8), 2.65(3H, s, CH3 acetyl), 2.52(3H, s, C2-CH3), and 2.49(3H, s, C7-CH3). |
| 2g | 7.89(1H, s, H-5), 7.18(1H, s, H-8), 2.66(3H, s, CH3 acetyl), 2.52(3H, s, C2-CH3), 2.42(3H, s, C7-CH3), and 2.35(3H, s, C6-CH3). |
| 2h | 9.97(1H, d, 3J=8.6, H-9), 8.06(1H, d, 3J=8.9, H-7), 7.85(1H, d, 3J=9.5, H-12), 7.68(1H, dd, 3J=8.6 and 6.9, H-10), 7.61(1H, dd, 3J=6.9 and 9.5, H-11), 7.45(1H, d, 3J=8.9, H-8), 2.70(3H, s, CH3 acetyl), and 2.52(3H, s, C2-CH3). |
| 2ib | 8.45(1H, d, 3J=7.5, H-9), 8.12(1H, d, 3J=8.7, H-5), 7.92(1H, d, 3J=6.8, H-12), 7.76(1H, d, 3J=8.7, H-6), 7.72(1H, d, 3J=7.5, H-10), 7.67(1H, d, 3J=6.8, H-11), 2.70(3H, s, CH3 acetyl), and 2.66(3H, s, C2-CH3). |
| 3c | 8,23-7.07(8H, m, arH); 2.52(3H, s) |
| 4b | 15.57(1H, s, 1OH); 11.87(1H, s, 2OH); 7.98-6.70(9H, m) |
| 4c | 15.48(1H, s, 1OH); 12.01(1H, s, 2OH); 8.02-7.44(9H, m) |
| 5a | 7.40-7.96(9H, m); 2.37(3H) |
| 5bx | 7.95-7.85(3H, m); 7.53-7.40(5H, s); 2.44(3H, s); 2.36(3H, s) |
| 5d | 8.56-7.43(11H, m); 2.20(3H, s) |
| 7a | 7.92(1H, s, OH); 7.45-6.76(7H, m); 4.71(2H, s, CH2-O-); 2.44(3H, CH3-); 2.39(3H, CH3) |
| 7b | 7.98(1H, s, OH); 7.48-7.26(5H, m); 6.99(1H, s); 7.74(2H, s, CH2-O); 2.44(3H, s); 2.40(3H, s) |
| 7c | 10.55(1H, s, OH); 8.44(1H, d, 3J 8.2 Hz); 6.99(1H, s); 7.64-6.60(7H, m); 4.66(2H, s, CH2O); 2.40(3H, s); 2.39(3H, s) |
| 8a | 7.97(1H, s, H-5); 7.49-6.95(5H, Ar-H); 5.40(2H, s, CH2O); 2.60(3H, s); 2.47(3H, s) |
| 8b insoluble | |
| 8c | 8.19(1H, s, H-5); 7.74-6.63(5H, m); 5.26(2H, s); 2.57(3H, s); 2.20(3H, s) |
| 8d | 7.95(1H, s); 7.48-7.15(5H, m); 5.99-5.90(1H, q, CH-O); 2.50(3H, s); 2.46(3H, s); 1.6(3H, d 3J=6.8Hz) |
| 8e | 7.99(1H, s, H-5); 7.59-6.60(5H, m); 5.29(2H, s, CH2-O); 2.56(3H, s); 2.46(3H, s); 2.21(3H, s) |
| 8f | 8.03(1H, s, H-5); 7.87-7.46(6H, m); 5.19(2H, s, CH2-N); 2.62(3H, s); 2.49(3H, s) |
| 9a | 11.58(1H, s, OH), 7.38(1H, s, H-6), 6.96(1H, s, H-3), 2.44(3H, s, CH3), 2.41(3H, s, CH3), and 2.32(3H, s, CH3). |
| 9b | 11.63(1H, s, OH), 7.19(1H, s, H-6), 6.86(1H, s, H-3), 2.32(3H, s, CH3), 2.30(3H, s, CH3), 2.28(3H, s, CH3), and 2.24(3H, s, CH3). |
| 9c | 11.95(14, s, OH); 7.65-6.55(9H, m); 2.33(3H, s) |
| 9d | 11.77(1H, s OH); 7.68-7.30(8H, m); 2.34(3H, s); 2.01(3H, s) |
| 9e | 11.81(1H, s OH); 7.62-6.9(8H, m); 2.33(3H, s) |
| 10a | 7.43(1H, s, H-5), 6.98(1H, s, H-8), 2.54(3H, s, CH3 acetyl), and 2.41(6H, brs, C2-CH3 and C7-CH3). |
| 10b | 7.18(1H, s, H-5), 6.85(1H, s, H-8), 2.50(3H, s, CH3 acetyl), 2.32(3H, s, C2-CH3), 2.27(3H, s, C7-CH3), and 2.22(3H, s, C6-CH3). |
| 10cx | 10.10(1H, s, OH); 7.72-6.60(9H, m); 2.26(3H,s) |
| 10dx | 9.87(1H, s, OH); 7.62-6.60(8H, m); 2.25(3H, s); 2.20(3H, s) |
| 10cx | 10.49(1H, s, OH); 7.68-6.53(8H, m); 2.25(3H, s) |
| 11a | 8.25(1H, s, H-2); 7.18-7.50(5H, m); 2.70(3H, s) |
| 11b | 8.25(1H, s, H-2); 8.50-8.10(2H, m); 7.78-7.39(7H, m); 2.69(3H, s); 2.65(3H, s) |
| 11c | 8.22(1H, s, H-2); 8.10-7.78(2H, m); 7.66-7.45(6H, m); 2.68(3H, s); 2.46(3H, s) |
| 11d | 8.23(1H, s, H-2); 8.32-8.03(2H, m); 7.80-7.42(7H, m); 2.68(3H, s); |
| 11e | 8.22(1H, s, H-2); 8.25-7.96(2H, m); 7.81-7.39(6H, m); 2.38(3H, s); 2.30(3H, s); 2.24(3H, s) |
| 11f | 8.24(1H, s, H-2); 8.32-7.40(7H, m); 2.69(3H, s); 2.39(3H, s); 2.34(3H, s) |
| Comp. | C-2 | C-3 | C-4 | C-4a | C-5 | C-6 | C-7 | C-8 | C-8a | CO acetyl | CH3 acetyl | CH3 |
| 2a | 168.5 | 123.6a | 175.7 | 123.8a | 125.5 | 125.8 | 133.9 | 117.6 | 155.2 | 200.3 | 32.1 | 19.7 |
| 2b | 168.3 | 123.3a | 175.9 | 123.4a | 125.1 | 135.5 | 135.2 | 117.4 | 153.5 | 200.5 | 32.1 | 20.9 19.7 |
| 2c | 168.8 | 123.6 | 174.7 | 124.7 | 125.3 | 131.5 | 134.2 | 119.5 | 153.6 | 200.0 | 32.2 | 19.8 |
| 2d | 168.7 | 123.6 | 174.4 | 125.0 | 128.5 | 118.9 | 136.9 | 119.6 | 154.0 | 199.8 | 32.0 | 19.7 |
| 2e | 168.9 | 124.0a | 174.0 | 125.6 | 124.0 | 131.2 | 134.0 | 123.6a | 149.7 | 199.3 | 32.0 | 19.7 |
| 2f | 168.6 | 123.4 | 174.6 | 122.7 | 125.5 | 132.2 | 143.3 | 119.5 | 153.5 | 200.1 | 32.1 | 20.8 19.8 |
| 2g | 168.0 | 123.3 | 175.7 | 121.4 | 125.3 | 134.7 | 144.4 | 117.7 | 153.7 | 200.7 | 32.1 | 20.3 19.7 19.2 |
| 2hb | 164.7 | 126.4 | 177.8 | 117.0 | 130.2 | 130.6 | 135.8 | 117.0 | 156.6 | 201.1 | 32.0 | 19.0 |
| 2ic | 167.4 | 124.6 | 175.7 | 123.5 | 120.5 | 125.6 | 135.9 | 120.1 | 152.7 | 200.5 | 32.2 | 19.7 |
© 1998 MDPI. All rights reserved. Molecules http://www.mdpi.org/molecules/
Share and Cite
Lacova, M.; El-Shaaer, H.M.; Loos, D.; Matulova, M.; Chovancova, J.; Furdik, M. Evaluation of Effect of Microwave Irradiation on Syntheses and Reactions of Some New 3-Acyl-methylchromones. Molecules 1998, 3, 120-131. https://doi.org/10.3390/30300120
Lacova M, El-Shaaer HM, Loos D, Matulova M, Chovancova J, Furdik M. Evaluation of Effect of Microwave Irradiation on Syntheses and Reactions of Some New 3-Acyl-methylchromones. Molecules. 1998; 3(3):120-131. https://doi.org/10.3390/30300120
Chicago/Turabian StyleLacova, Margita, Hafez M. El-Shaaer, Dusan Loos, Maria Matulova, Jarmila Chovancova, and Mikulas Furdik. 1998. "Evaluation of Effect of Microwave Irradiation on Syntheses and Reactions of Some New 3-Acyl-methylchromones" Molecules 3, no. 3: 120-131. https://doi.org/10.3390/30300120




