Results and Discussion
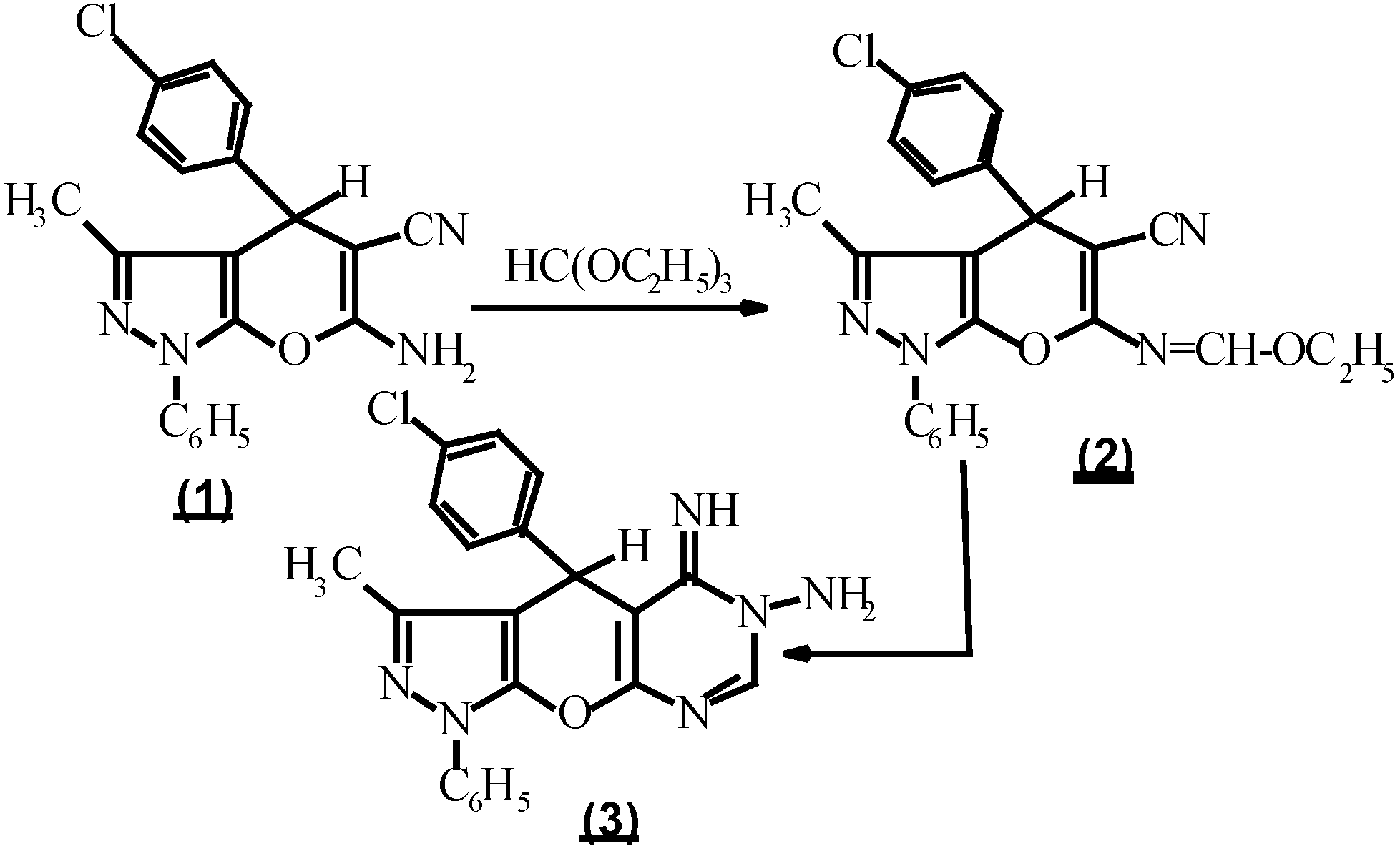
In the present study, we report on the synthesis of pyrazolo [4`, 3` : 5,6] pyrano [3,2-e] [1,2,4]-triazolo [1,5-c] pyrimidine and pyrazolo [4``, 3`` : 4`,5`] pyrano [2` 3` : 4,5] pyrimido [1,6-b] [1,2,4]-triazine derivatives via the reaction of 6-amino-1,4,5,6-tetrahydro-5-imino-3-methyl-1-phenyl-4-(p-chlorophenyl) pyrazolo [4`, 3` : 5,6] pyrano [2,3-d] pyrimidine (3) with different reagents.
Compound
3, considered as a key intermediate to prepare fused heterocycles as triazolo [1,5-c] pyrimidines which may possess pharmacological properties similar to theophylline [
7,
8], was prepared by treating ethyl N-(5-cyano-1,4-dihydro-3-methyl-1-phenyl-4-(p-chlorophenyl)-pyrano-[2,3-c] pyrazol-6-yl-methan-imidate (
2) with hydrazine hydrate [
9,
10]. On the other hand, compound
2 was prepared by the reaction of 6-amino-5-cyano-1,4-dihydro-3-methyl-1-phenyl-4 (p-chlorophenyl) pyrano [2,3-c] pyrazole (
1) [
11,
12] with an equimolar amount of triethyl orthoformate and acetic anhydride [
9,
10] (
Scheme 1).
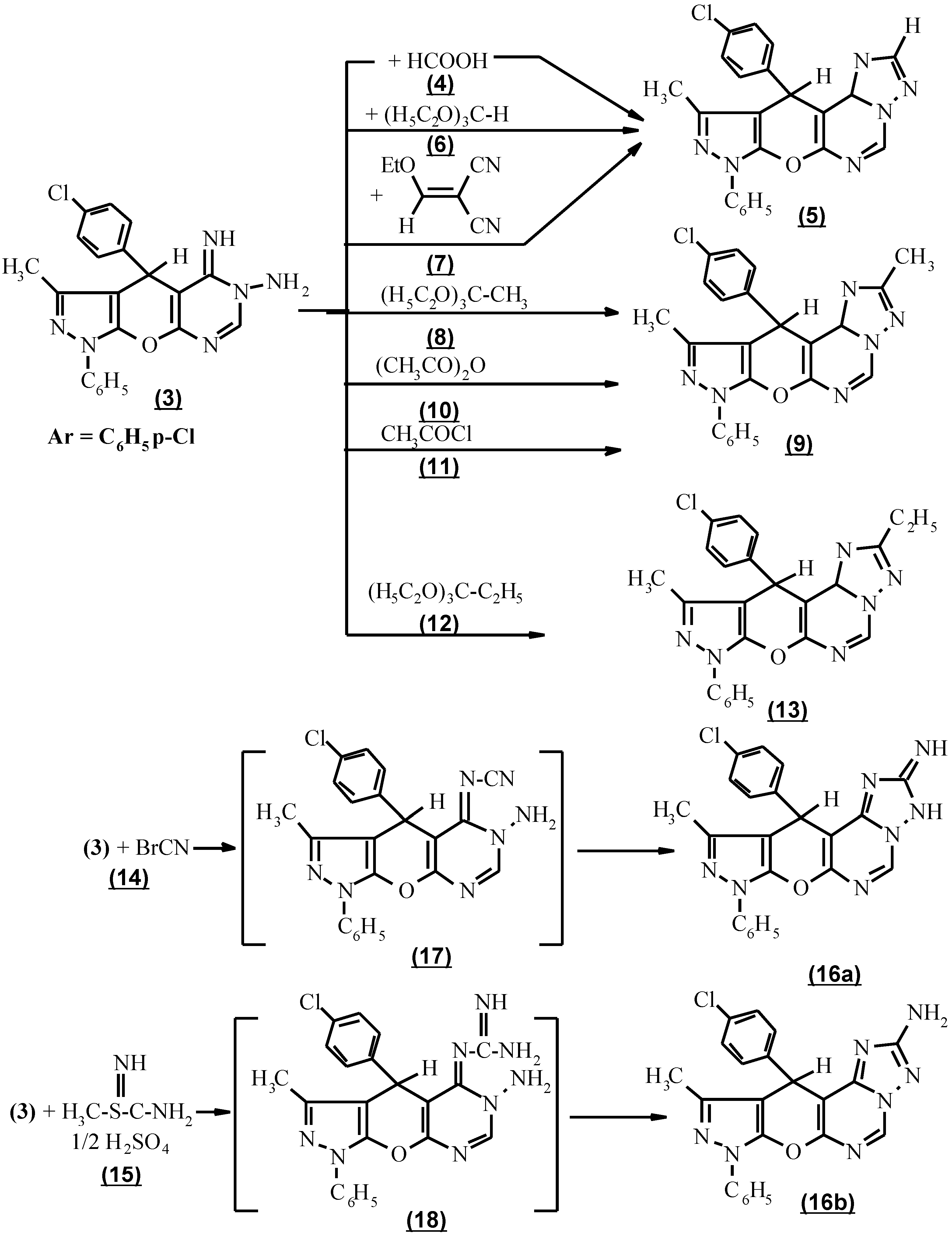
The cyclocondensation of compound
3 with the appropriate carboxylic acid derivatives was performed by heating compound
3 with an excess of neat formic acid
4 to afford 8,11-dihydro-10-methyl-8-phenyl-11-(p-chlorophenyl) [4`,3` : 5`,6`] pyrano [3,2-e] [1,2,4]-triazolo [1,5-c] pyrimidine (
5) in a 65% combined yield. An improvement in the preparation of this compound was achieved using triethylorthoformate (
6) by refluxing neat at 140°C, or by reacting of compound (
3) with ethoxymethelyene malononitrile (
7) to afford 80-82% yield (
Scheme 2).
When triethylorthoacetate (
8) was used in the above cyclocondensation, 3,10-dimethyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl pyrazolo [4`,3`: 5,6] pyrano [3,2-e]-[1,2,4] triazolo [1,5-c] pyrimidine (
9) was produced. Compound
9 was also produced via the reaction of
3 with acetic anhydride (
10) as carboxylic acid anhydride and acetyl chloride (
11) as acid chloride. The structure of
9 was based on the absence of NH and NH
2 absorption in the i.r. spectrum. (
Scheme 2).
Moreover, the interaction of triethylorthopropionate (12) with 3 afforded 3-ethyl -10-methyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl-pyrazolo [4`, 3` : 5,6] pyrano [3,2-e] [1,2,4] triazolo [1,5-c] pyrimidine (13).
The subsequent incorporation of a one- carbon unit carrying an amino group into
3 to form the triazolo component was performed via two facile procedures. The first one consisted in applying cyanogen bromide (
14) condensation to compound
3 and the second one involved refluxing the compound
3 and S-methyl isothiourea sulfate (
15) [
13] to afford 3-amino-10-methyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl pyrazolo-[4`, 3` : 5,6] pyrano [3,2-e] [1,2,4] triazolo [1,5-c] pyrimidine (
16b).That compound
16b exists in the 2-amino tautomeric form and not in the 2-iminoform was shown by the appearance of an intense absorption band at 3400 cm
-1 and a two proton singlet as broad band at 3.7 ppm due to NH
2 in the i.r. and
1H NMR spectra respectively. In the case involving the condensation of compound
3 with cyanogen bromide (
14) and S-methyl isothiourea sulfate (
15), the intermediates formed might bear a cyanimino (
17) or guanidino function (
18). These intermediates (
17 and
18) were cyclized ) in an alkaline medium to give the target molecule
16b as expected (
Scheme 2). However because of the different electronic influence on each reaction centre,
16b was obtained in different yields.
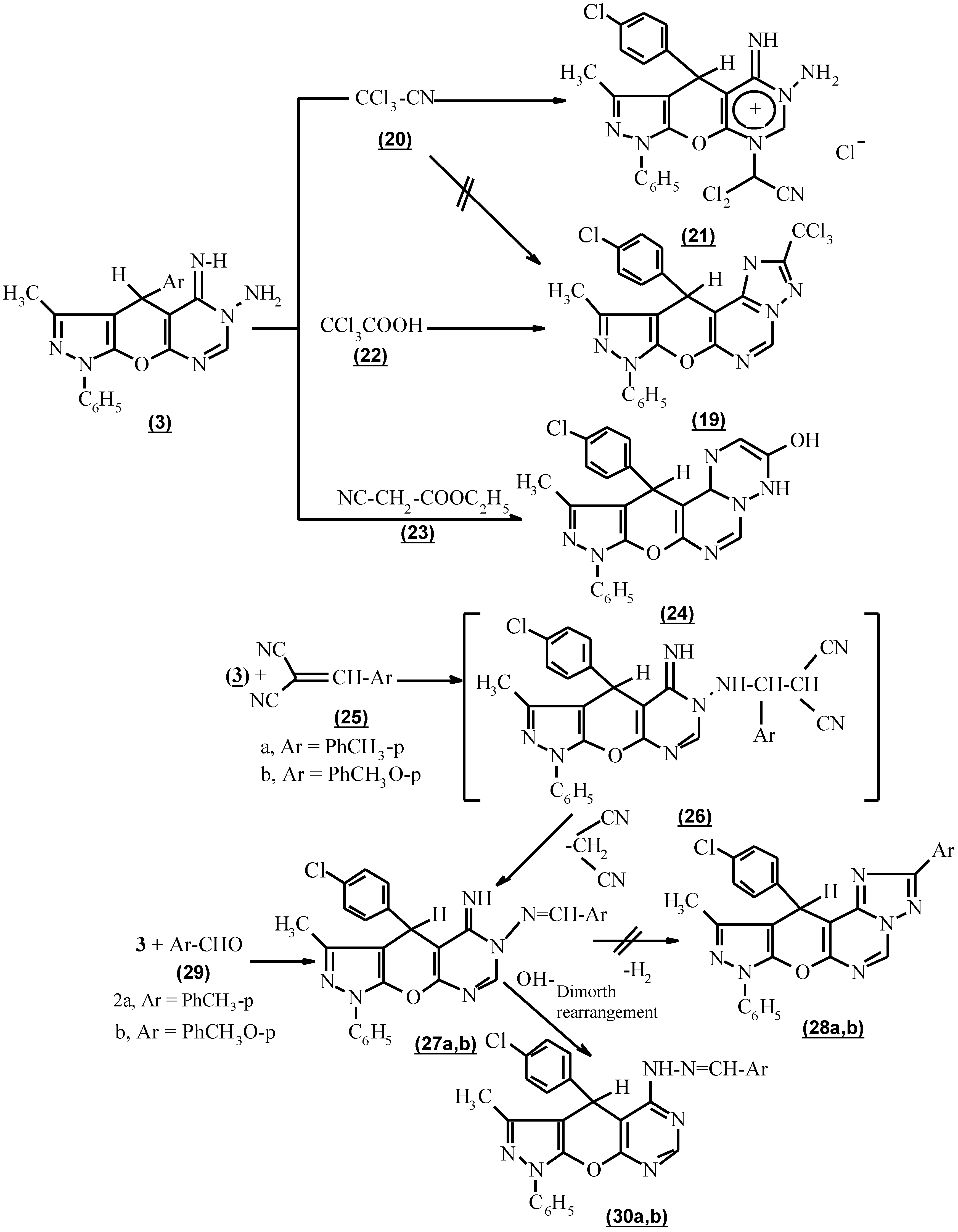
The formation of 3-trichloromethyl-10-methyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl pyrazolo [4`, 3` : 5,6] pyrano [3,2-e] [1,2,4] triazolo [1,5-c] pyrimidine (
19) via the interaction of compound 3 with trichloroacetonitrile (
20) in the absence of solvent under reflux according to a reported procedure for the construction of an allied heterocyclic system [
14] was unsuccessful (
Scheme 3). The pyrimidinium salt (
21) was formed [15,16a,b], based on the presence of NH and NH
2 in its i.r. spectrum and the appearance of CN, its mass spectrum was (M
+ - NH
2, 532.2, 30%). The trichloro methyl derivatives (
19) were achieved using trichloroacetic acid (
22) in the presence of phosphoryl chloride under reflux for 3 hours. The structure (
19) was confirmed by the absence of NH and NH
2 absorption in its i.r. spectrum.
The activity of compound
3 towards the reaction product was active methylene was also tested [
17]. Thus, with ethyl cyanoacetate (
23), 9,12-dihydro-3-hydroxy-11-methyl-9-phenyl-12-(p-chlorophenyl) 2H-pyrazolo [4``, 3`` : 5`,6`] pyrano [2`,3`:5,6] pyrimido [1,6-b] [1,2,4] triazine (
24) as confirmed by its spectral data. (
Scheme 3).
Compound
3, when reacted with β-cyanocinnamonitrile derivatives, namely p-tolylmalononitrile and p-anisylmalononitrile (
25a,b) respectively, in dioxane under reflux and in the presence of a catalytic amount of piperidine failed to afford 8,11-dihydro-11-(p-chlorophenyl)-3-aryl-8-methyl-10-phenylpyrazolo [4`,3` : 5,6] pyrano [3,2-e] [1,2,4] triazolo [1,5-c] pyrimidine (
28a,b). In addition p-tolylmalononitrile and p-anisylmalononitrile (
25a,b) respectively reacted with compound
3 to give 6-aryl methylene hydrazono-1,4,5,6-tetrahydro-3-methyl-1-phenyl-4-(p-chlorophenyl) pyrazolo-[4`, 3` : 5,6] pyrano [3,2-d] pyrimidine (
27a,b) via the formation of 1,1 adduct (
26a,b) followed by the loss of malononitrile [
18]. Also the same product (
27b) was isolated from the reaction of compound
3 with p-tolulaldehyde and
p-anisaldehyde
29a,b respectively. Based on their spectral data no cyclization took place (
Scheme 3). From this experiment, it can be concluded that a Taylor-Löeffler transformation has occured [
9]. Because the reaction was carried out in basic medium as catalyst which facilitates the Dimorth rearrangement, the formal structure
30a,b is preferable (
Scheme 3).
For convenience of the readers, the spectroscopic data are also collected in
Table 1.
Experimental
General
Melting points are uncorrected and were taken on a Boetius melting point microscope. Microanalyses were performed by the Micro-analytical unit Cairo University I.R. spectra were recorded on a Mattson 5000 FIR spectrometer 1H NMR spectra were determined on a Varian EM NMR spectrometer using tetra-methylsilan as an internal standand. Mass spectra (MS) were recorded on a Finigan SSQ 7000 mass spectrometer.
Ethyl N-(5-cyano-1,4-dihydro-3-methyl-1-phenyl-4-(p-chlorophenyl) pyrano[2,3-c] [pyrazol -6-yl] methanimidate (2):
A mixture of 1 (1.79 g, 0.005 mol) triethylorthoformate (0.741 g, 0.005 mol) and acetic anhydride (16 ml) was refluxed for 5 h. The solvent was removed under reduced pressure and the resulting solid product is crystallized from benzene to give 1.84 g (92%) of 2 as colourless needles. M.p. 166-168°C. 1H NMR (DMSO-d6) δ 1.5 (t,3H, CH3), 2.00 (s, 3H, CH3), 4.41 (q, 2H, CH2), 4.9 (s, 1H, H-4), 7.45-7.84 (m, 9H, arom.) 8.3 (s, 1H, CHOEt). IR (kBr) 2220 (CN). Anal. Calcd. for C23H19N4OCl; C; 58.57; H, 4.75 Found: C; 68.44; H 4.59.
6-Amino-1,4,5,6-tetrahydro-5-imino-3-methyl-1-phenyl-4-(p-chlorophenyl) pyrazolo [4`,3`, :5,6] pyrano [2,3-d] pyrimidine (3).
To a solution of 2 (2g, 0.005 mol) in methanol (25 ml) a solution of hydrazine hydrate (5 ml) was added and the mixture stirred for 1 hr. Then it is allowed to stand overnight. The precipitate formed is filtered, dried and crystallized from methanol to afford. 55% of 3. M.p. 213-214°C: 1H NMR (DMSO-d 6): δ 1.95 (s, 3H, CH3), 5.00 (s, 1H, H-4), 5.6 (s, 1H, NH), 7.45-7.84 (m, 9H, arom.), 8.3 (s, 1H, H-7). IR: 3370, 3340 (NH2), 3150(NH). MS m/z (%), 404 (20) M+, 388 (60) 174(100), 77(65). Anal. Calcd. for C21H17N6OCl; C, 62.30; H, 4.23, Found C, 62.19, H; 4.18.
8,11-Dihydro-10-methyl-8-phenyl-4-(p-chlorophenyl) pyrazolo [4`,3`: 5,6] pyrano [3,2-e] [1,2,4] triazolo [1,5-c] pyrimidine (5).
Method A
A mixture of 3 (1.01g, 0.0025 mol) and (32 ml, 0.7 mol) of formic acid 4 was refluxed for 10 hrs. and then cooled poured onto ice-water to give a white precipitate which was filtered, washed several times by water, dried. and then crystallized from ethanol to afford 55% of 5. M.p. 324-325°C. 1H NMR (DMSO-d6): δ 1.9 (s, 3H, CH3) 5.7 (s, 1H, H-11), 7.4-7.8 (m, 9H, arom.), 8.65 (s, 1H, H-2), 9.7 (s, 1H, H-5). MS: m/z (%) 414 (47) M+, 303 (100), 278 (25), 77 (20). Anal. Calcd. for C22H15N6OCl; C; 63.69, H; 3.64 Found C; 63.49, H; 3.49.
Method B
A solution of 3 (1.01 g; 0.0025 mol) and (2.2 g, 0.02 mole) of triethylorthoformate 6 neat was refluxed for 8 hrs. A precipitate was formed, filtered while and recrystallized from ethanol to give 0.83g (80%) of 5.
Method C
To a solution of 3 (1.01g, 0.0025 mol) in absolute ethanol (30 ml) was added ethoxymethylene malononitrile 7 and the mixture was refluxed for 6 hr (TLC control). On cooling, a solid product separated, which was filtered and recrystallized from ethanol to give 0.85g (82%) of 5.
3,10-dimethyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl pyrazolo [4`,3`,: 5,6] pyrano [3,2-e] [1,2,4,] triazolo [1,5-c] pyrimidine (9)
Method A
A solution of 3 (1.01g, 0.0025 mol) and (3.3g 0.2 mol) of triethylorthoacetate (8), neat was refluxed for 8 hrs, a precipitate was formed, filtered while hot, and recrystallized from ethanol to give 0.75g (70%) of 9. M.p. 264-265°C. 1H NMR (DMSO-d6): δ 1.9(s, 3H, CH3), 2.3 (s, 3H, CH3) 5.6 (s, 1H, H-11), 7.4-7.8 (m, 9H, arom.) 9.6 (s, 1H, H-5). MS, m/z (%) 428 (25) M+, 317 (100) 77 (17). Anal. Calcd. for C23H17N6OCl; C: 64.41, H; 3.99, Found C. 64.32 H, 3.79.
Method B
A solution of 3 (1.01g, .0025 mol) and 10 ml of acetic anhydride (10) was refluxed for 10 hrs. A precipitate was formed, filtered while hot, and recrystallized from ethanol to give 0.8g (75%) of 9.
Method C
A mixture of 3 (1.01g, 0.0025 mol) and 7 ml of acetyl chloride (11) in 10 ml dimethylformamide was heated for 4 hrs on a water bath and after cooling, poured into 100 ml of ice water. The precipitate was collected on a filter, washed with water and recrystallized from ethanol to give 0.9g (84%) of 9.
3-Ethyl 10-methyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl pyrazolo [4`,3`:5,6]-pyrano[3,2-e][1,2,4]triazolo [1,5-c] pyrimidine (13).
A solution of 3 (1.01 g, 0.0025 mol) and (3.4 g, 0.02 mol) of triethy-lor-thopropionate 12 neat was refluxed for 8 hrs, and then worked out as described above to produce 0.83 g (75%) of 13. M.p. 310-312°C. 1H NMR (DMSO-d6): δ 1.35 (t, 3H, CH3), 1.95 (s, 3H, CH3), 3.4 (q, 2H, CH2), 5.6 (s, 1H, H-11) 7.4-7.8 (m, 9H, arom.), 9.5(s, 1H, H-5). MS: m/z (%) 428 (M+, 28), CH3, 317 (100). Anal. Calcd. for C24H19N6OCl, C: 65.08, H, 4.32, Found C: 64.89, H, 4.22.
3-amino-10-methyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl pyrazolo [4`,3`:5,6] pyrano [3,2-e] [1,2,4] triazolo [1,5-c] pyrimidine(16b).
Method A
A solution of 3 (1.01 g, 0.0025 mol) in 50 ml dimethyl-formamide was kept at 0°C and treated with cyanogen bromide 14 (0.6 g, 0.005 mol). The mixture was refluxed for 5 hrs, then cooled and poured onto water to give a white precipitate, filtered, washed with water and crystallized from dimethylformamide to yield 0.54 g (50%) of 16b. M.p.>300 C. 1H NMR (DMSO-d6): δ 1.6 (s, 3H, CH3), 3.6 (br, 2H, NH2 exchangeable with D2O), 6.2 (s, 1H, H-11) 7.3-7.8 (m, 9H, arom.), 9.3(s, 1H, H-5), I.R.: 3178, 3320 (NH2). MS: m/z (%) 429 (M+, 37), 388 (57), 318 (60), 278 (100). Anal. Calcd. for C22H16N7OCl, C: 61.47, H; 3.75, Found C: 61.37, H; 3.66.
Method B
A solution of 3 (1.01 g, 0.0025 mol) and (0.28 g, 0.001 mol) of S-methylisothiourea sulfate 15 in alcoholic potassium hydroxide (0.5 N) was heated under reflux for 8 hours. After cooling, the mixture was poured on to water to give a white precipitate, which was collected, washed with water and recrystallized from dimethylformamide to yield 0.6 g (60%) of 16b.
3-trichloromethyl-8,11-dihydro-11-(p-chlorophenyl)-8-phenyl pyrazolo [4`,3`:5.6] pyrano[3,2-e] [1,2,4] triazolo [1,5 -c] pyrimidine (19)
A mixture of 3 (1.01 g, 0.0025 mol) and 0.49 g (0.003 mol) of trichloroacetic acid (22) in 15 ml of freshly distilled phosphorus oxychloride, was refluxed on a water bath for 4 hrs, cooled then poured onto ice -water to give a precipitate which was filtered off, and washed with water. The solid that collected by filteration, was recrystallized in dioxane to afford 0.7 g (51%) of (19). m.p. 270-271°C. 1H NMR (DMSO-d6): 1.8 (s, 3H, CH3), 4.6 (s, 1H, H-11), 7.2-7.7 (m, 9H, arom.) 8.4 (s, 1H, H-pyrimidine). Anal., Calcd. for C23H14N6Ocl4 C: 51.91, H: 2.65, Found C: 51.81, H: 2.61.
9,12-dihydro-3-hydroxy-11-methyl-9-phenyl-12-(p-chlorophenyl)-2H-pyrazolo [4``,3``:5`,6`] pyrano [2`,3`:4.5] pyrimido [1,6-b] [1,2,4] triazine (24)
A mixture of (3) (1.01 g, 0.0025 mol) andethylcyanoacetate (23) (0.1 ml, 0.001 mol) in absolute ethanol (50 ml) was refluxed for 10 hr. A precipitate was formed, filtered off washed with ethanol, and recrystallized from dioxane to afford 0.78 g (70%) of (25). m.p. 276-277° C. 1H NMR (DMSO-d6): 1.95 (s, 3H, CH3), 4.4 (s, 1H, NH), 5.6 (s, 1H, H-12), 7.4-7.8 (m, 9H, arom.) 8.4 (s, 1H, H-2), 9.5 (s, 1H, H-6). IR: 3500 (OH, NH). MS m/z (%), 414 (40) (M+ -CH3,OH), 303 (100). Anal., Calcd. forC23H17N6O2Cl C: 62.09, H: 3.85, Found C: 61.89, H: 3.66.
5-arylmethylene hydrazono-1,4,5,6-tetrahydro-3-methyl-1-phenyl-4-(p-chlorophenyl) pyrazolo [4`,3`:5.6] pyrano [2,3 d] pyrimidine (30a,b).
Method A
A mixture of 3 (1.01 g, 0.0025 mol) and p-tolylmalononitrile (25a) or p-anisylmalononitrile (25b) (0.0025 mol) in dioxane, in the presence of piperidine as a catalyst was refluxed for 8 hours. After cooling, the solid product formed was collected and crystallized from dioxane to yield 70% and 80% of 30a,b respectively.
Method B
A mixture of 3 (1.01 g, 0.0025 mol) and p-tolulaldehyde (29a) or p-anisaldehyde (29b) (0.0025 mol) in dioxane in the presence of piperidine as a catalyst was refluxed for 8 hours and then worked out as described above to produce. 76%, 85% of (30a,b).
30a: m.p. 184-186°C. 1H NMR: 1.9 (s, 3H, CH3) 2.4 (s, 3H, CH3), 5.9 (s, 1H, H-11), 7.3-7.5 (m, 13H, arom.) 8.2 (s, 1H, N=CH), 8.5 (s, 1H, H-pyrimidine), 10.6 (br, 1H, NH, D2O exchangeable). IR: 1600 (C=N), 3450 (NH). MS m/z (%), 506 (M+, 14), 393 (40), 386, 100, 77 (15). Anal., Calcd. for C29H23N6OCl; C: 68.70, H: 4.57, Found C: 68.60, H: 4.49.
30b: m.p. 196-197°C. 1H NMR: 1.9 (s, 3H, CH3), 3.4 (s, 3H, OCH3), 5.9 (s, 1H, H-11), 7.3-7.8 (m, 13H, arom.) 8.4 (s, 1H, N=CH), 8.56 (s, 1H, H-pyrimidine), 10.8 (br, 1H, NH, D2O exchangeable). IR: 1620 (C=N), 3450 (NH). Anal., Calcd. for C29H23N6O3Cl C: 66.60, H: 4.43, Found C: 66.49, H: 4.33.