Synthesis of 2-(6-Acetamidobenzothiazolethio)acetic Acid Esters as Photosynthesis Inhibitors
Abstract
:Introduction
Results and Discussion
f = 0.958 s = 0.1874 F = 33.6 n = 9
f = 0.9799, s = 0.13040, F = 72.6,
n = 9, logP0 = 2.71, β= 1.1690.10-3
Experimental
General
Results of 1H NMR analysis (80 MHz, deuterated DMSO)
6-Acetamidobenzothiazole skeleton
2-(6-Acetamidobenzothiazolethio)acetic Acid Esters 1 -13
Acknowledgements:
References
- Sidoova, E.; Odlerova, Z.; Volna, F.; Blockinger, G. Chem. Zvesti 1979, 33, 830.
- Sidoova, E.; Kuchta, T.; Zemanova, M.; Strakova, H. CS Pat. 261699, 1988.
- Kuchta, T.; Strakova, H.; Sidoova, E. Cs. Farmacie 1989, 38, 139.
- Kuchta, T.; Bujdakova, H.; Sidoova, E. Folia Microbiologica 1989, 34, 504.
- Kuchta, T.; Bujdakova, H.; Sidoova, E.; Neslusanova, H. Acta Fac. Rerum. Natur. Univ. Comen. Microbiologia 1990, 17–18, 49.
- Bujdakova, H.; Kuchta, T.; Sidoova, E. Acta Fac. Rerum. Natur. Univ. Comen. Microbiologia 1991, 19–20, 37.
- Kralova, K.; Loos, D.; Sersen, F.; Sidoova, E. Chem. Papers 1994, 48, 198.
- Sidoova, E.; Odlerova, Z. Chem. Papers 1990, 44, 375.
- Sidoova, E.; Odlerova, Z. Chem. Papers 1985, 39, 553.
- Kuchta, T.; Sidoova, E. Cs. Farmacie 1989, 38, 310.
- Kralova, K.; Sersen, F.; Sidoova, E. Chem. Papers 1992, 46, 348.
- Sidoova, E.; Perjessy, A.; Loos, D.; Kuchta, T. Chem. Papers 1992, 46, 420.
- Kralova, K.; Bujdakova, H.; Kuchta, T.; Loos, D. Pharmazie 1994, 49, 460.
- Sersen, F.; Kralova, K.; Sidoova, E. Photosynthetica 1993, 29, 147.
- Kralova, K.; Sersen, F.; Sidoova, E. Gen. Physiol. Biophys. 1993, 12, 421.
- Dewar, M. J. S.; Zoebisch, E.; Healy, E.; Stewart, J. P. P. J. Am. Chem. Soc. 1985, 107, 3902. [CrossRef]
- Ertl, P. Chem. Listy 1992, 86, 465.
- Kubyni, H. Arzneim-Forsch. (Drug Res.) 1976, 26, 1991.
- Kubyni, H.; Kehrhahn, O. H. Arzneim-Forsch. (Drug Res.) 1978, 28, 598.
- Kralova, K.; Sersen, F.; Mitterhauszerova, L.; Devinsky, F.; Krempaska, E. Photosynthetica 1992, 26, 181.
- Samples Availability: Samples are available from the authors and MDPI.
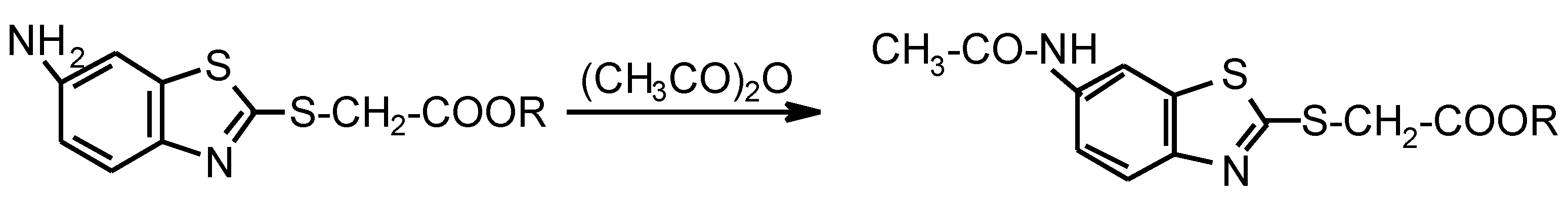

| Comp. R | Formula Mr | C | Wi(calc.) %/ Wi(found) % H N S | Yield % | M.p. (°C) | |||
| 1 | -CH3 | C12H12N2O3S2 | 48.63 | 4.08 | 9.45 | 21.64 | 94.5 | 154.5-155.5 |
| 296.37 | 48.40 | 4.02 | 9.26 | 21.65 | ||||
| 2 | -C2H5 | C13H14N2O3S2 | 50.30 | 4.55 | 9.03 | 20.66 | 99.8 | 131.0-132.5 |
| 310.40 | 50.53 | 4.59 | 9.04 | 20.80 | ||||
| 3 | -(CH2)2CH3 | C14H16N2O3S2 | 51.83 | 4.97 | 8.63 | 19.77 | 92.5 | 111.5-112.5 |
| 324.42 | 51.65 | 5.03 | 8.63 | 19.99 | ||||
| 4 | -CH2CH=CH2 | C14H14N2O3S2 | 52.16 | 4.38 | 8.69 | 19.89 | 86.8 | 119-120 |
| 322.41 | 52.49 | 4.36 | 8.43 | 20.19 | ||||
| 5 | -CH2C≡CH | C14H12N2O3S2 | 52.48 | 3.78 | 8.74 | 20.02 | 90.5 | 123.0-124.5 |
| 320.39 | 52.29 | 3.66 | 8.77 | 19.73 | ||||
| 6 | -(CH2)3CH3 | C15H18N2O3S2 | 53.23 | 5.36 | 8.28 | 18.95 | 74.0 | 113-114 |
| 338.45 | 53.42 | 5.37 | 8.34 | 18.97 | ||||
| 7 | -CH(CH3)C2H5 | C15H18N2O3S2 | 53.23 | 5.36 | 8.28 | 18.95 | 88.6 | 117-118 |
| 338.45 | 53.02 | 5.41 | 8.16 | 18.83 | ||||
| 8 | -(CH2)4CH3 | C16H20N2O3S2 | 54.52 | 5.72 | 7.95 | 18.19 | 85.2 | 116.5-118.5 |
| 352.48 | 54.55 | 5.74 | 7.92 | 18.09 | ||||
| 9 | -(CH2)5CH3 | C17H22N2O3S2 | 55.71 | 6.05 | 7.64 | 17.49 | 79.1 | 77-79 |
| 366.50 | 56.03 | 6.21 | 7.64 | 17.44 | ||||
| 10 | -(CH2)6CH3 | C18H24N2O3S2 | 56.81 | 6.36 | 7.36 | 16.85 | 47.4 | 82.5-84.5 |
| 380.53 | 57.04 | 6.44 | 7.31 | 16.88 | ||||
| 11 | -(CH2)7CH3 | C19H26N2O3S2 | 57.84 | 6.64 | 7.10 | 16.25 | 38.0 | 71.5-73.5 |
| 394.56 | 58.06 | 6.74 | 7.07 | 16.25 | ||||
| 12 | -(CH2)8CH3 | C20H28N2O3S2 | 58.79 | 6.91 | 6.86 | 15.70 | 39.2 | 80-81 |
| 408.59 | 58.50 | 6.92 | 6.76 | 15.63 | ||||
| 13 | -CH2-C6H5 | C18H16N2O3S2 | 58.04 | 4.33 | 7.52 | 17.22 | 99.3 | 122.5-124.0 |
| 372.47 | 58.09 | 4.42 | 7.48 | 17.43 | ||||
| Compound | logP | IC50 (μmol dm-3) |
| 1 | 0.86 | - |
| 2 | 1.20 | 857 |
| 3 | 1.67 | 514 |
| 4 | 1.60 | 411 |
| 5 | 1.14 | 350 |
| 6 | 2.07 | 83 |
| 7 | 2.09 | - |
| 8 | 2.46 | 56 |
| 9 | 2.86 | 47 |
| 10 | 3.26 | 106 |
| 11 | 3.65 | 430 |
| 12 | 4.05 | 1879 |
| 13 | 2.64 | 622 |
© 1998 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sidoova, E.; Kralova, K.; Loos, D. Synthesis of 2-(6-Acetamidobenzothiazolethio)acetic Acid Esters as Photosynthesis Inhibitors. Molecules 1998, 3, 135-140. https://doi.org/10.3390/30400135
Sidoova E, Kralova K, Loos D. Synthesis of 2-(6-Acetamidobenzothiazolethio)acetic Acid Esters as Photosynthesis Inhibitors. Molecules. 1998; 3(4):135-140. https://doi.org/10.3390/30400135
Chicago/Turabian StyleSidoova, Eva, Katarina Kralova, and Dusan Loos. 1998. "Synthesis of 2-(6-Acetamidobenzothiazolethio)acetic Acid Esters as Photosynthesis Inhibitors" Molecules 3, no. 4: 135-140. https://doi.org/10.3390/30400135





