A New Isoflavone from Smilax glabra
Abstract
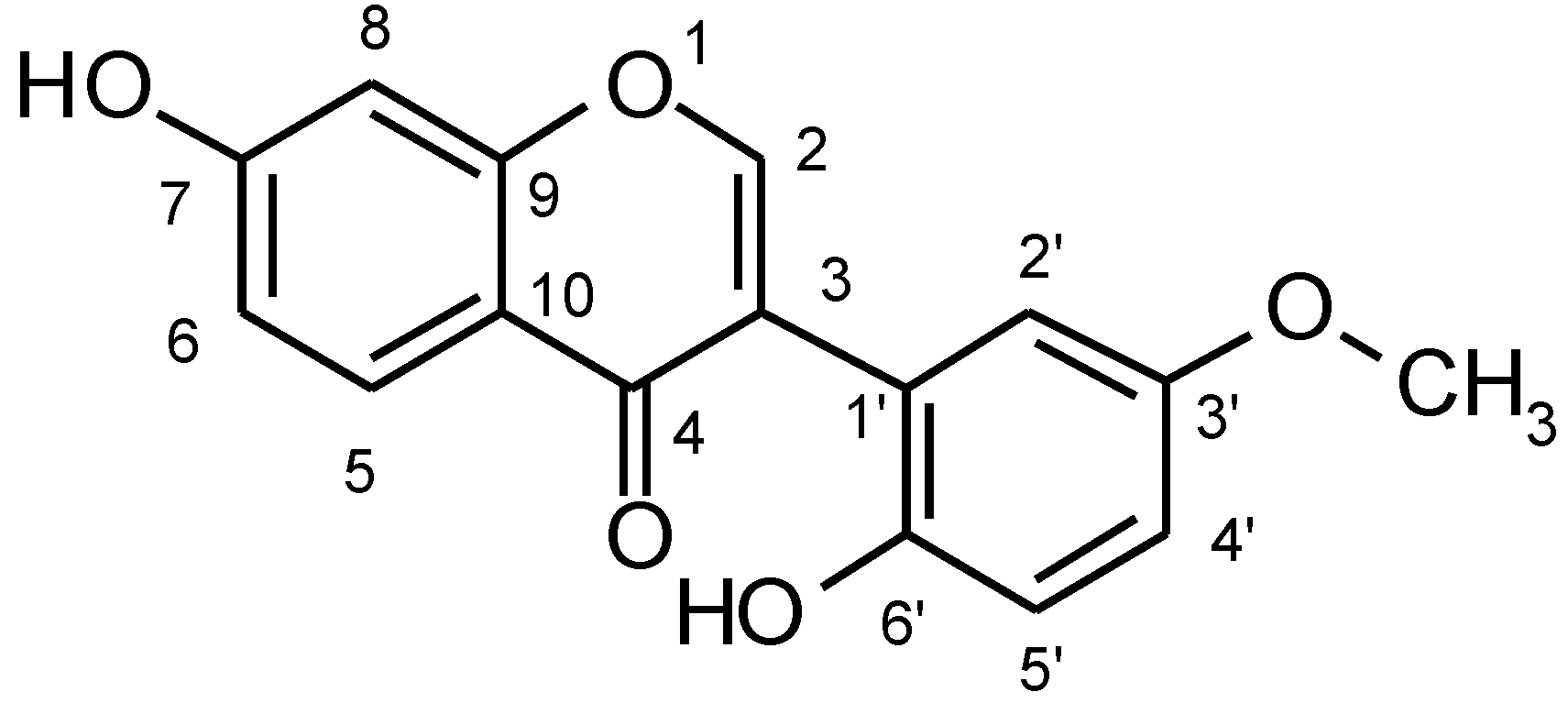
:Introduction

Results and Discussions
Experimental Section
Acknowledgement
References and Notes
- Jiangsu New College of Medicine. A Dictionary of Traditional Chinese Drugs; People’s Press: Shanghai, 1977; pp. 91–93. [Google Scholar]
- Cao, Z. Z.; Yi, Y. J.; Hong, W. Q.; Ling, Y. L. Zhongcaoyao 1993, 24, 234.
- Cao, Z. Z.; Yi, Y. J.; Yang, D. L. Natural Product R & D 1994, 6(2), 33.
- Cao, Z. Z.; Yi, Y. J.; Cao, Y.; Leng, Z. K. Chin. Chem. Lett. 1995, 6, 587.
- Yi, Y. J.; Cao, Z. Z.; Yang, W. H.; Hong, W. Q.; Cao, Y.; Leng, Z. K. Yaoxue Xuebao 1995, 30, 718.
- Sample Availability: available from MDPI.
| Atomic no. | δC | CHn | δH | Mult. | J(Hz) | COLOC (C to H) | 1H-1H COSY |
|---|---|---|---|---|---|---|---|
| 2 | 153.4 | CH | 7.7 | s | H-2 | H-2’, CH3 | |
| 3 | 125.0 | C | H-2,H-2’ | ||||
| 4 | 175.0 | C | H-5, H-2,H-8 | ||||
| 5 | 127.6 | CH | 7.4 | d | 8.7 | H-5 | H-6 |
| 6 | 115.5 | CH | 6.3 | m | H-8,OH-7 | H-5, H-8 | |
| 7 | 162.9 | C | H-5, H-8 | ||||
| 8 | 102.4 | CH | 6.1 | d | 2.0 | OH-7, H-8,H-6 | H-5,H-6 |
| 9 | 157.7 | C | H-5, H-2, H-8 | ||||
| 10 | 117.0 | C | H-6, H-8 | ||||
| 1’ | 123.0 | C | H-2’,H-2,H-5’ | ||||
| 2’ | 120.1 | CH | 6.5 | d | 2.0 | H-2’,H-4’ | H-2, H-4’ |
| 3’ | 147.8 | C | CH3, H-2’,H-4’ | ||||
| 4’ | 116.8 | CH | 6.3 | m | H-2’,H-5’ | CH3 ,H-2’ | |
| 5’ | 112.2 | CH | 6.3 | m | H-4’ | H-2’ | |
| 6’ | 146.3 | C | H-2’,H-5’ | ||||
| OMe | 55.9 | CH3 | 3.8 | s | CH3 | H-2, H-4’ |
© 1998 MDPI. All rights reserved. Molecules http://www.mdpi.org/molecules/
Share and Cite
Yi, Y.; Cao, Z.; Yang, D.; Cao, Y.; Wu, Y.; Zhao, S. A New Isoflavone from Smilax glabra. Molecules 1998, 3, 145-147. https://doi.org/10.3390/30500145
Yi Y, Cao Z, Yang D, Cao Y, Wu Y, Zhao S. A New Isoflavone from Smilax glabra. Molecules. 1998; 3(5):145-147. https://doi.org/10.3390/30500145
Chicago/Turabian StyleYi, Yijun, Zhengzhong Cao, Dalong Yang, Yuan Cao, Yongping Wu, and Shouxun Zhao. 1998. "A New Isoflavone from Smilax glabra" Molecules 3, no. 5: 145-147. https://doi.org/10.3390/30500145




