Fluorescent 2,7-Dialkylamino-[1,8]-Naphthyridines: Preparation and Spectroscopic Properties
Abstract
:Introduction
Results and Discussion
Syntheses of 2.7-dialkylamino-4-methyl-[1.8]-naphthyridines
Fluorescence and UV Behaviour
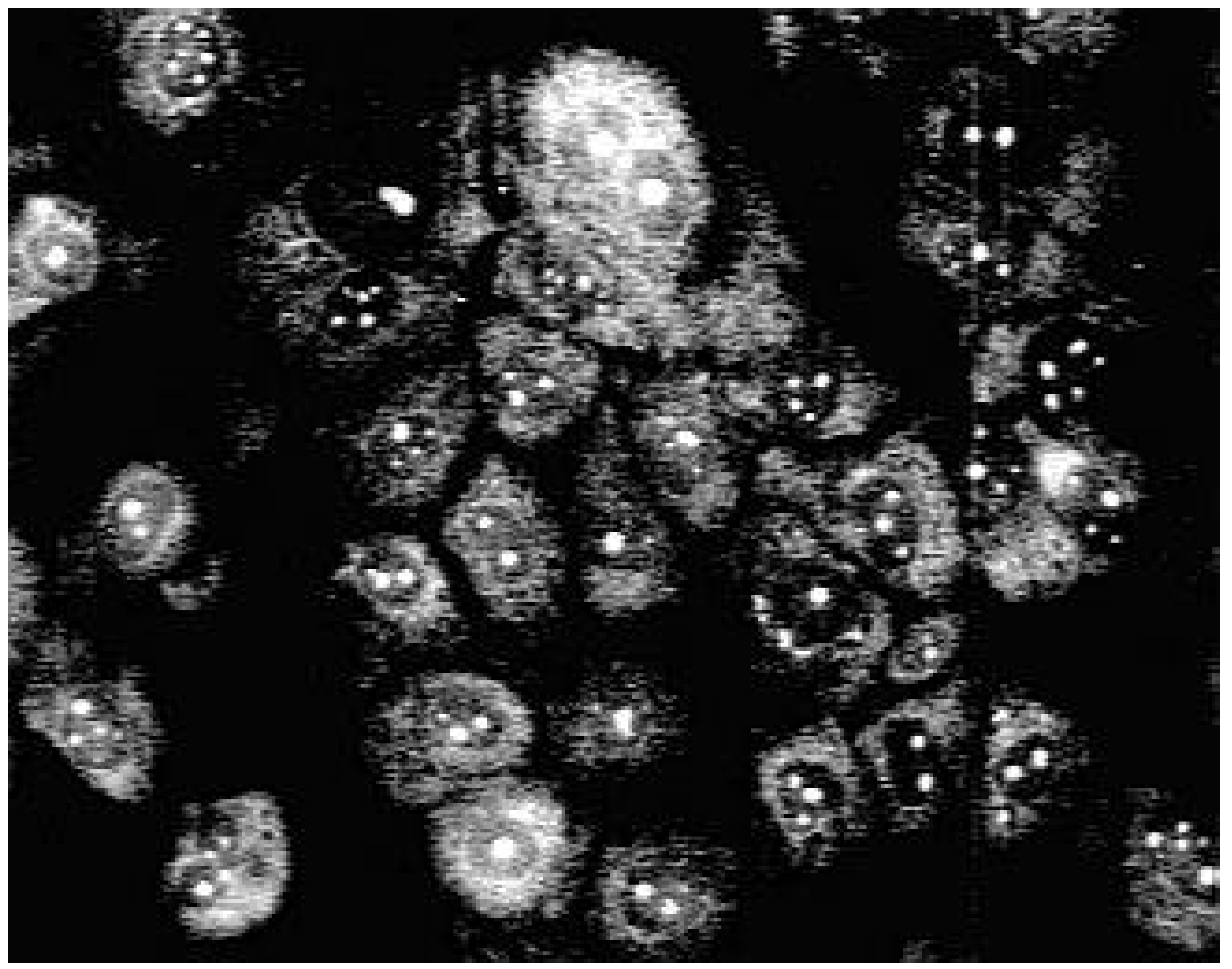
Biological use
Experimental
General
2,7-Dimethylamino-4-methyl-[1.8]-naphthyridine 3a
2,7-Dipropylamino-4-methyl-[1.8]-naphthyridine 3b
2,7-Dibutylamino-4-methyl-[1.8]-naphthyridine 3c
N2,N7-Bis-(2-methoxy-ethyl)-4-methyl-[1,8]naphthyridine-2,7-diamine 3d
2,7-Dihexylamino-4-methyl-[1.8]-naphthyridine 3e
Acknowledgement
References and Notes
- a) Kricka, L.J. Nonisotopic DNA Probe Techniques; Acedemic Press: New York, 1992. [Google Scholar] ; b) Symons, R.H. Nucleic Acid Probes; CRC Press, 1989. [Google Scholar] ; c) Connolly, B.A.; Newman, P.C. Nucleic Acids Res. 1989, 17, 4957–4971.
- Drexhage, K.H.; Erikson, G.R.; Hawks, G.H.; Reynolds, G.A. Opt. Commun. 1975, 15, 399–403. b) Drexhage, K.H.; Reynolds, G.A. Opt. Commun. 1973, 13, 222–225. c) Drexhage, K.H. USP 3 873 940/1971.
- Fletcher, A.N. Appl. Phys. 1977, 14, 295–302.
- Hammond, P.R.; Atkins, R.L.; Henry, R.A.; Fletcher, A.N. USP 4 103 257/1976.
- Staubli, A.B.; Dervan, P.B. Nucleic Acids Res. 1994, 22, 2637–2642.
- Meier, T. Ph.D. Thesis, University of Konstanz Germany, 1995.
- Seide, O. Chem. Ber. 1926, 59, 2465–2473.
- Petrow, V.; Rewald, E.L.; Sturgeon, B. J. Chem. Soc. 1947, 1407–1411. [CrossRef]
- Mangini, A.; Colonna, M. Gazz. Chim. Ital. 1942, 72, 190–195.
- a) Bergstrom, F.W. J. Org. Chem. 1946, 11, 239–246. b) Wozniak, M.; Skiba, M. Pol. J. Chem. 1981, 55, 2429–2437.
- Ochiai, E.; Miyaki, K. Chem. Ber. 1941, 74, 1115–1126, ref. 9.
- Carboni, S.; Settimo, A.; Bertini, D.; Ferrarini, P.; Livi, O.; Tonetti, I. Il Farmaco 1975, 30, 185–196.
- Hammond, P.R.; Fletcher, A.N.; Henry, R.A.; Atkins, R.L. Appl. Phys. 1975, 8, 315–318.
- Obtained from Aldrich.
- Haugland, J. Handbook of Fluorescent Probes; Mol. Ed. Probes Inc.: Junction City, Oregon, 1986. [Google Scholar]
- Methods used for our experiments refer to Ploem, J.S. Fluorescence microscopy. In Light Microscopy – A Practical Approach; Lacey, A.J., Ed.; IRL Press: Oxford, 1989; p. 137, and references cited herein. [Google Scholar]
- Schmidtke, M.; Reichert, J.; Hoock, C. Unpublished work.
- Schmidtke, M.; Knorre, C.; Blei, L.; Stelzner, A.; Birch-Hirschfeld, E. Nucleosides, Nucleotides 1998, 17, 1557–1566.
- Samples Availability: Samples available from the authors.
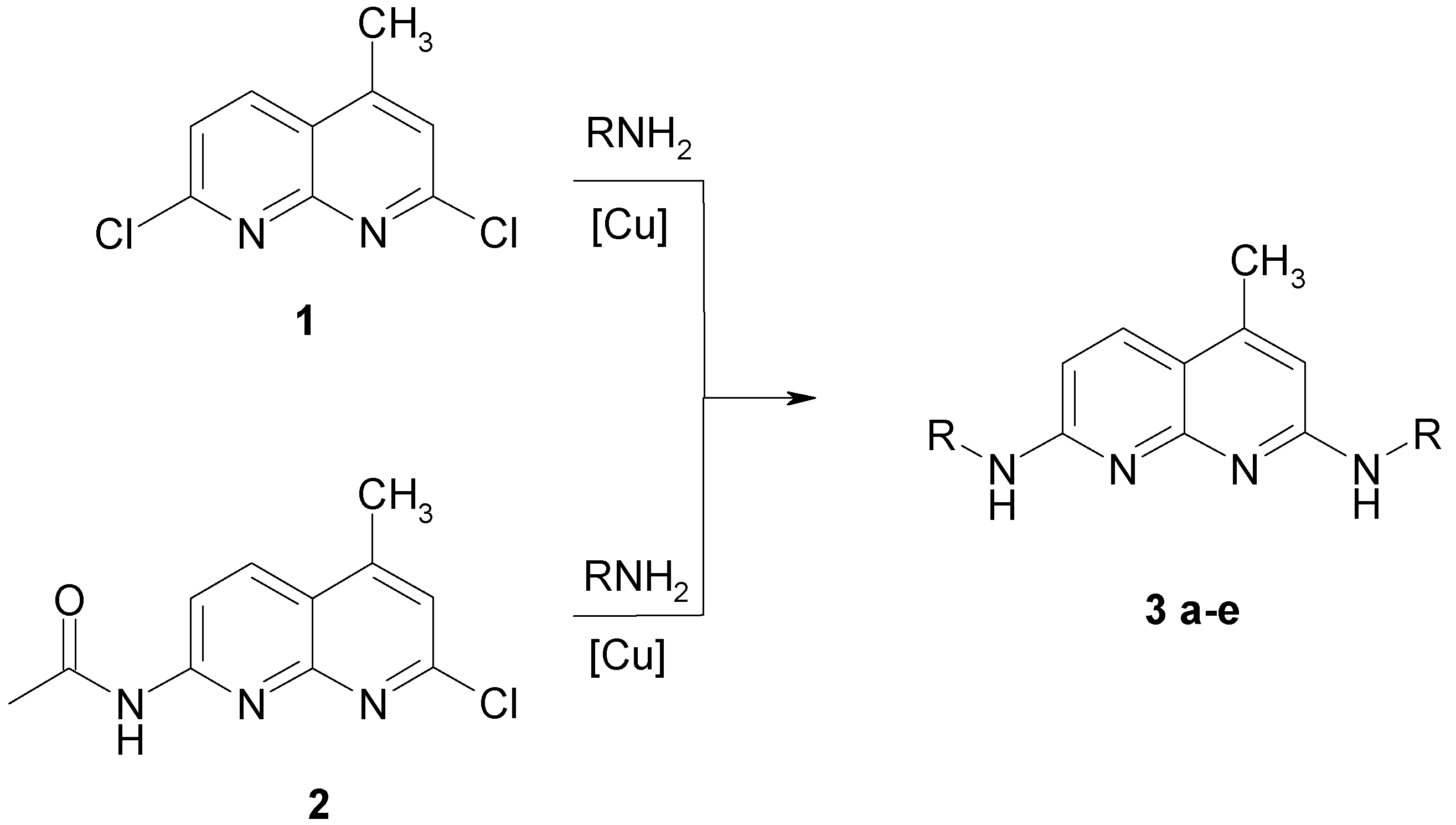

| Nr. | R | Yield [%]a | Rf | Φ | |
| 1 | 2 | ||||
| 3a | CH3 | n. a.b | 34 | 0.20 | 0.25 |
| 3b | n-C3H7 | 49 | 18 | 0.46 | 0.19 |
| 3c | n-C4H9 | 65 | 22 | 0.55 | 0.28 |
| 3d | n-C2H4-O-CH3 | 20 | 22 | 0.26 | 0.24 |
| 3e | n-C6H13 | 65 | 2 | 0.78 | 0.26 |
© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.
Share and Cite
Hoock, C.; Reichert, J.; Schmidtke, M. Fluorescent 2,7-Dialkylamino-[1,8]-Naphthyridines: Preparation and Spectroscopic Properties. Molecules 1999, 4, 264-271. https://doi.org/10.3390/41000264
Hoock C, Reichert J, Schmidtke M. Fluorescent 2,7-Dialkylamino-[1,8]-Naphthyridines: Preparation and Spectroscopic Properties. Molecules. 1999; 4(10):264-271. https://doi.org/10.3390/41000264
Chicago/Turabian StyleHoock, Christoph, Jörg Reichert, and M. Schmidtke. 1999. "Fluorescent 2,7-Dialkylamino-[1,8]-Naphthyridines: Preparation and Spectroscopic Properties" Molecules 4, no. 10: 264-271. https://doi.org/10.3390/41000264




