8-Chloro-11-[4-(8-chloro-5H-dibenzo[b,e][1,4]diazepin-11-yl)piperazino]-5H-dibenzo[b,e][1,4]diazepine
Abstract
:Introduction
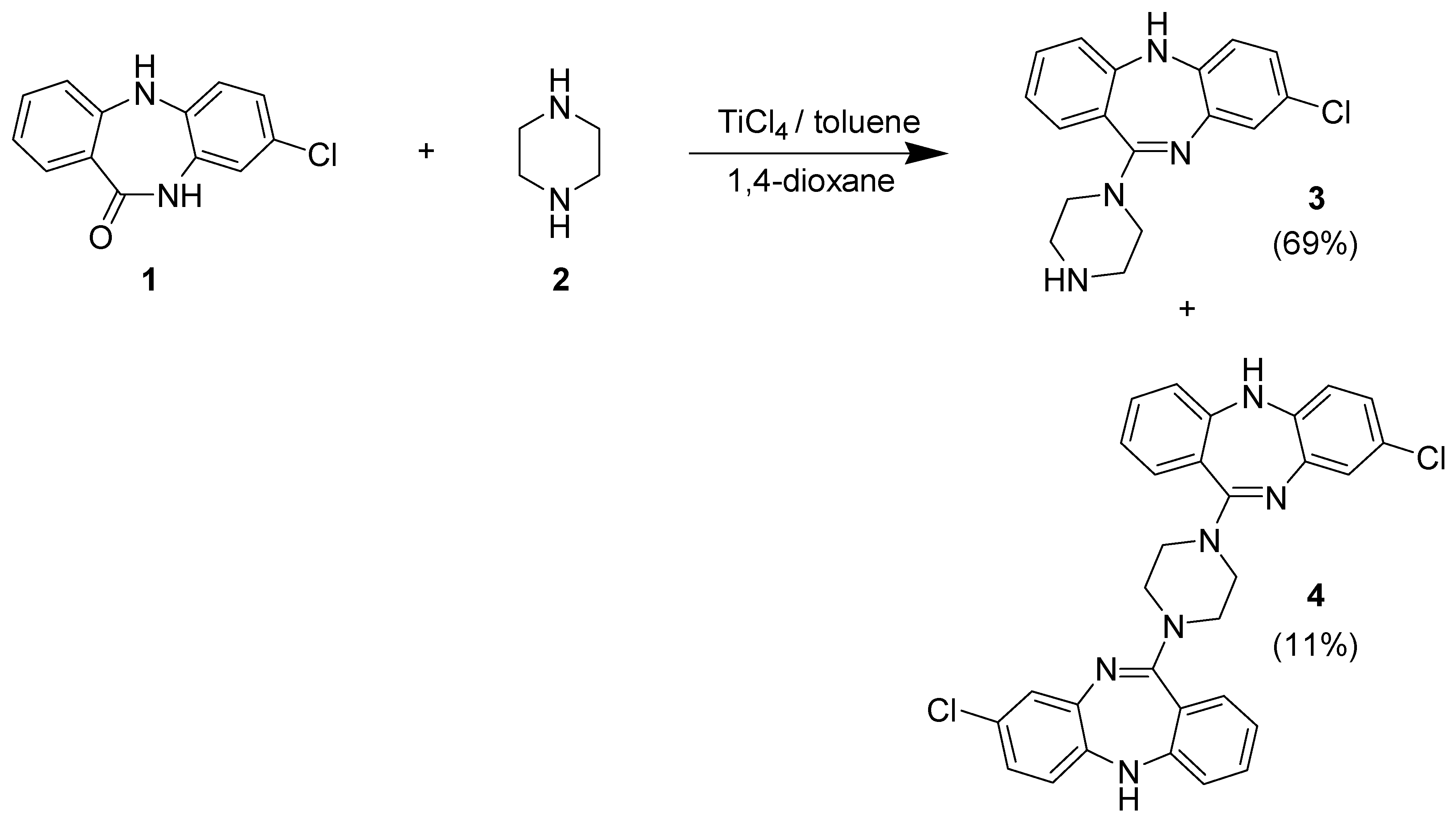
Results and Discussion
Conclusion
Experimental
General
Method
Spectral Data for Compound 4
Acknowledgment
References and Notes
- Gerson, S. L.; Arce, C.; Meltzer, H. Y. N-desmethylclozapine: a clozapine metabolite that suppresses haemopoiesis. Br. J. Haematol. 1994, 86, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J. Neues Verfahren zur Herstellung von organischen Verbindungen. Ger. Patent No. 2 316 438, 1973. [Google Scholar]
- Hunziker, F.; Fischer, E.; Schmutz, J. 11-Amino-5H-dibenzo[b,e]-1,4-diazepine. Helv. Chim. Acta 1967, 50, 1588–1599. [Google Scholar] [CrossRef]
- Samples Availability: available from MDPI.
© 1999 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Capuano, B. 8-Chloro-11-[4-(8-chloro-5H-dibenzo[b,e][1,4]diazepin-11-yl)piperazino]-5H-dibenzo[b,e][1,4]diazepine. Molecules 1999, 4, 329-332. https://doi.org/10.3390/41100329
Capuano B. 8-Chloro-11-[4-(8-chloro-5H-dibenzo[b,e][1,4]diazepin-11-yl)piperazino]-5H-dibenzo[b,e][1,4]diazepine. Molecules. 1999; 4(11):329-332. https://doi.org/10.3390/41100329
Chicago/Turabian StyleCapuano, Ben. 1999. "8-Chloro-11-[4-(8-chloro-5H-dibenzo[b,e][1,4]diazepin-11-yl)piperazino]-5H-dibenzo[b,e][1,4]diazepine" Molecules 4, no. 11: 329-332. https://doi.org/10.3390/41100329



