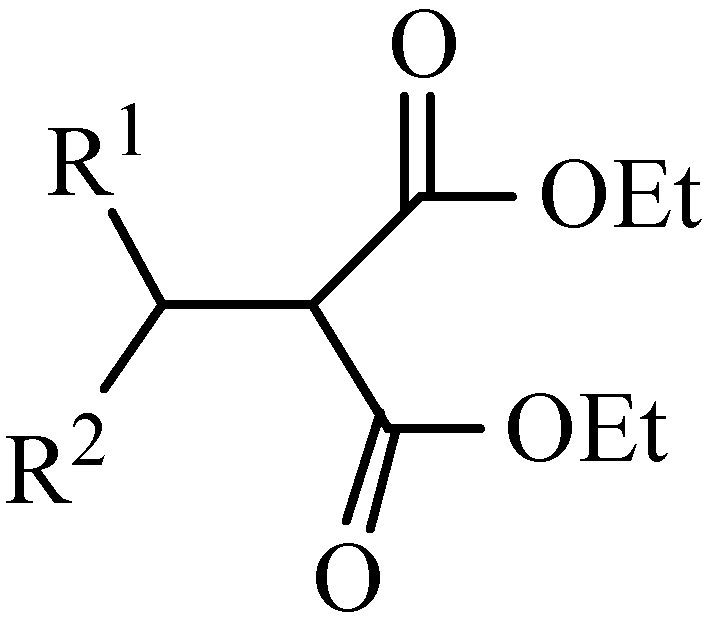
Introduction
Diethyl ethoxymethylene malonate (EMME) is an extremely valuable starting material for the synthesis of quinolones [
1]. Our continuing interest in model studies aimed at discerning the mode of action of quinolone antibacterials [
2] led us to prepare several arylidene malonic acids. These compounds (
1-6) were prepared by the reaction of EMME with the appropriate Grignard reagent. A method for protection of the amino moiety in amino acids using EMME was reported by Alaiz et. al., [
3] but most examples of these type of compounds were prepared by conjugate addition of various aniline derivatives to EMME [
4]. The products of this latter reaction were the corresponding β-amino diethylmethylene malonates. There have also been reports of the conversion of EMME to benzo[b]quinolizines via reaction with oxoquinolinyl acetates [
5], and into pyrido[
2,3-d]pyrimidines by reaction with oxopyrimidines [
6]. In contrast to these previous reports, we have found that the reaction of EMME and Grignard regents or organocuprates gives symmetric malonates
1-
4, or asymmetric malonate derivatives
5-
6 in moderate yield. Given the synthetic interest in this class of compounds, a collection of mass spectral fragmentation patterns to assist in the structural identification of these compounds would be useful.
Results and Discussion
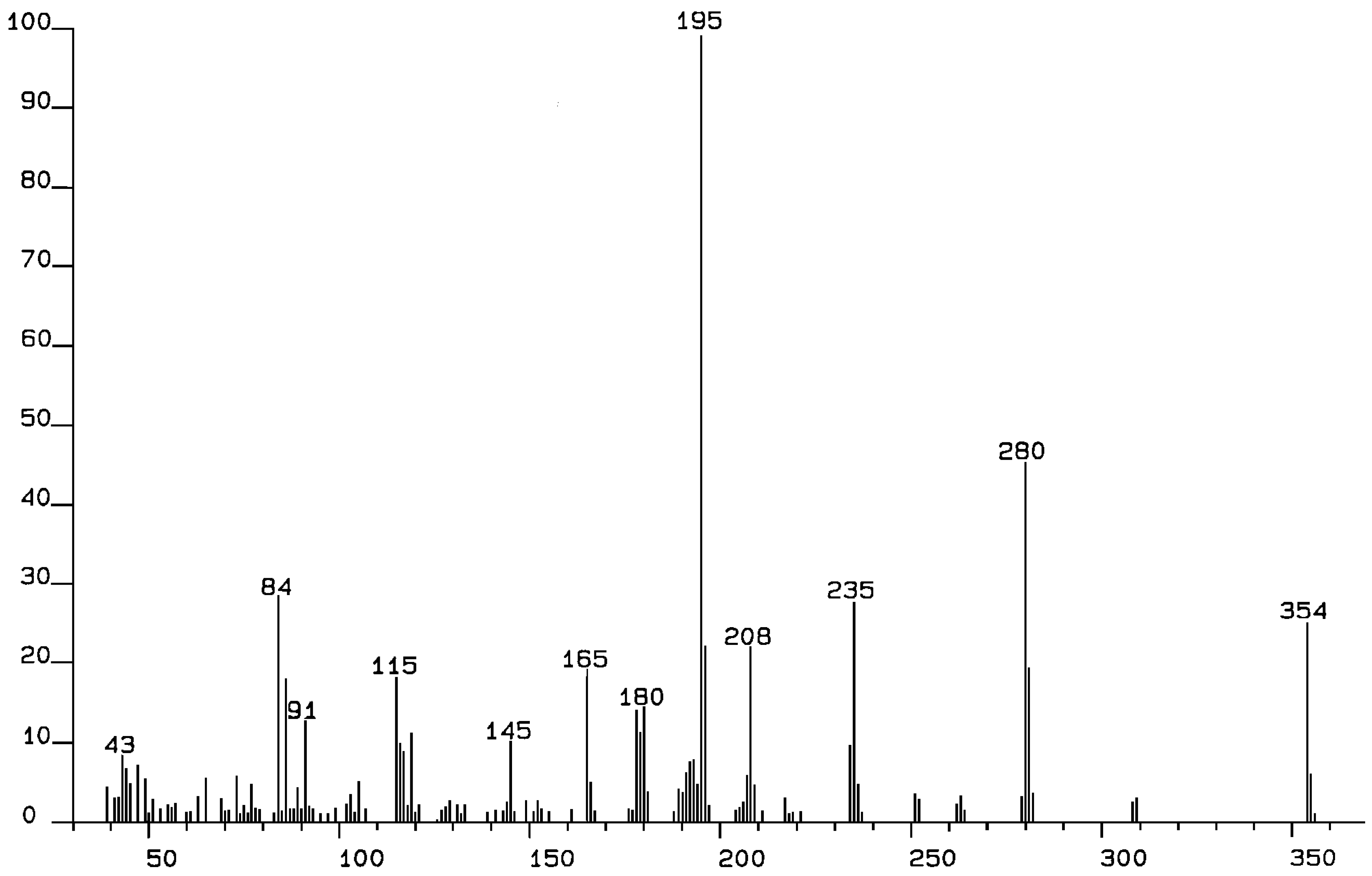
We report the electron impact mass spectra of 1-6, recorded at 70 eV. Aryl derivatives (1-4) give an intense molecular ion. This is in contrast to the asymmetric phenyl malonate (5) and the n-butyl derivative (6), which gave no apparent molecular ions. Facile loss of the phenyl and n-butyl moieties probably give a stable ion, whereas the benzylic nature of 1-4 leads to stable ions. The most important fragmentation is the loss of the diethyl malonate moiety (M-159). Indeed, this is the base ion for 1, 3 (m/z, 203) and 5 (m/z, 135). Although it does not lead to the base ion for 2, 4, or 6, this fragmentation gives a very intense ion at m/z 195, 195, 115 respectively.
The first striking feature of these molecules is seen by comparing 1, 2, and 4, which give remarkably different mass spectral fragmentations. Clearly, this system is very sensitive to the substituents attached to the malonate moiety. The most obvious fragmentation for 1, 2, and 4 is loss of the malonate group (M-159) to give the analogous “doubly benzylic” ion: m/z 195 (100), m/z 195 (82), and m/z 195 (74) respectively. Not surprisingly, fluorophenyl derivative 3 also shows this fragmentation, to give m/z 203(100). Clearly, this is a major fragmentation mode and leads to the base ion for both 1 and 3. What is remarkable, is the difference between 1 and 2. The base ion for 2 is m/z 115 (100) but m/z 115 for 1 has only 15% relative intensity. The only structural difference is the presence of a para-methyl in 1 and an ortho-methyl in 2. A reasonable explanation is that the ortho-methyl allows a transfer of a hydrogen atom to C2 of the malonate moiety, allowing expulsion of diethyl malonate as a neutral molecule (m/z 160). This neutral molecule shows no ion but loss of OEt (mass 45) gives the m/z 115 ion (base peak). Since the intramolecular transfer of a hydrogen atom from methyl is not possible in 1 (with a para-methyl group), this simple structural difference leads to a profoundly different fragmentation pattern. In both 1 and 2 the m/z 91 ion (tropylium) results from cleavage of the methylbenzene moiety. Interestingly, this ion does not appear in the spectrum of 4. Malonate 4 has a bis-benzyl moiety attached to the connecting carbon, giving two phenethyl groups. Loss of malonate gives the m/z 195 ion mentioned above, but with a relative intensity of 74%. The m/z 159 fragment (malonate - H) then loses an ethyl group (M-29) and also an OEt group (M-45) to give a fragment at m/z 85 (96) which probably correlates with [O=C-CH-CO2]. Loss of one hydrogen atom gives m/z 84 (100), the base ion, which would correlate with a ketene unit [O=C=C-CO2]. Once again, modification of the substituents leads to a very different fragmentation pattern. The fluorine atoms in 3 lead to yet another fragmentation pattern. The base ion at m/z 203 (100) dominates the fragmentation but loss of CO2Et (M-73) leads to an ion at m/z 289 (20).
Comparing 1-4 with the ethoxy derivatives 5-6 leads to the observation of very different fragmentation patterns. The symmetrical bis-aryl derivatives all show a parent ion of moderate intensity. This contrasts with 5 and 6, which show no parent ion at all. Once again, loss of malonate (M-159) from 5 or 6 leads to an ion at m/z 135 (100) for 5 and at m/z 115 (72) for 6. There is no significant ion for loss of OEt (M-45) from the parent ions in either case and the phenyl group in 5 is not lost. In 6, however, a weak ion for loss of OEt [m/z, 229 (7)] and a moderate intensity ion for loss of butyl [m/z 217 (20)] are apparent. The base ion in 6 is at m/z 59, which is likely due to fragmentation of the m/z 115 daughter ion.
Conclusion
It is clear that malonate derivatives such as 1-6 show a characteristic β-cleavage (β- to the carbonyl groups) with loss of malonate. The remainder of the fragmentation ions, however, are very characteristic of the substituents at C2 of the malonate. In many cases, these fragmentations form almost a “fingerprint” pattern rather than a general identification pattern. None-the-less, the fundamental "malonate-cleavage" mode can be used for identification purposes.
Experimental Section
The mass spectra were recorded on a Kratos MS 25 RFA instrument at an ionization voltage of 70 eV, using the direct probe. All the commercially available compounds were purified by chromatography on silicagel and were pure when analyzed using 1H NMR, 13C NMR, and infrared spectroscopy.