Furyl(aryl)alkanes and Their Derivatives. 19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring Closure Type
Abstract
:Introduction
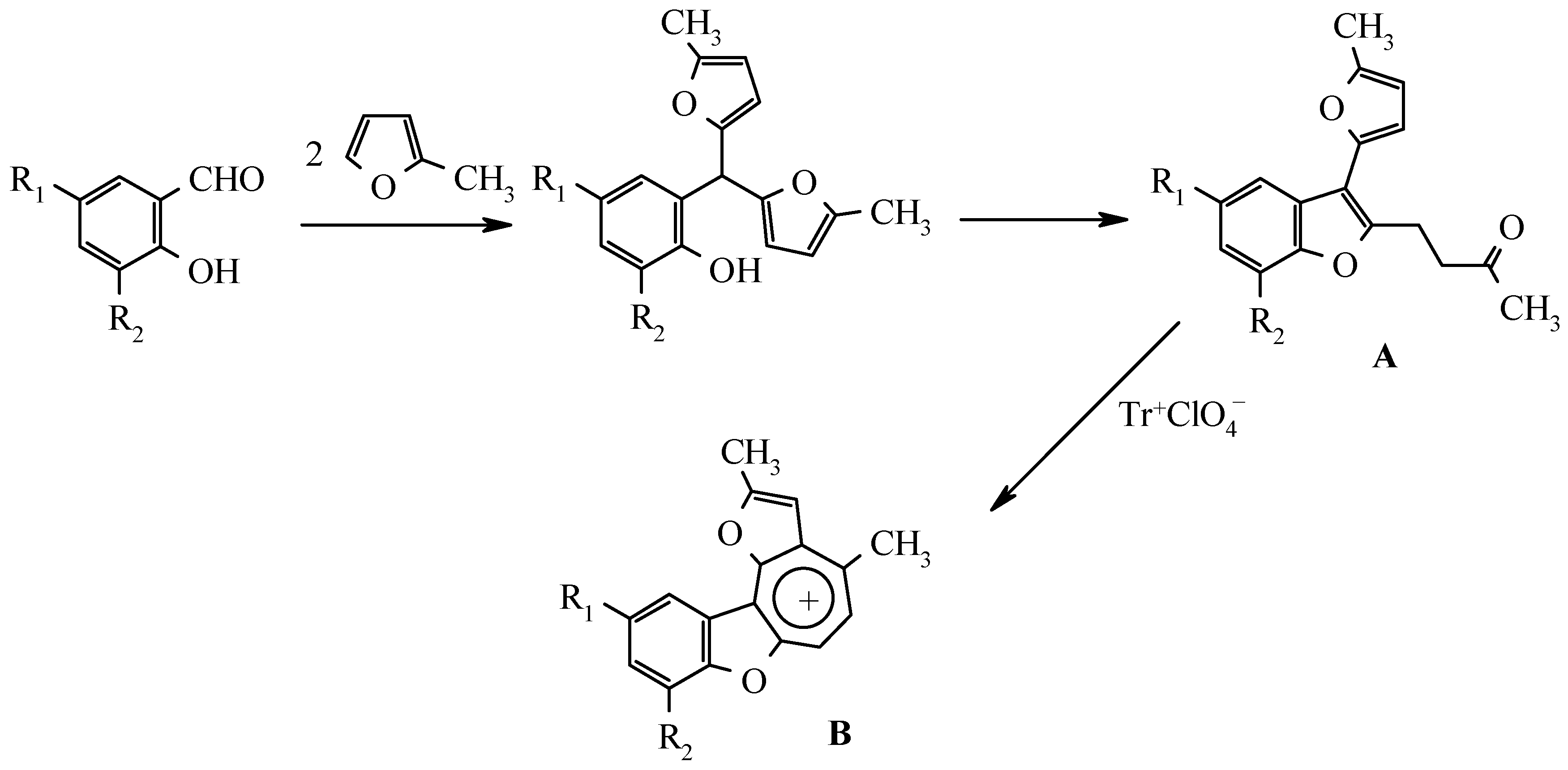
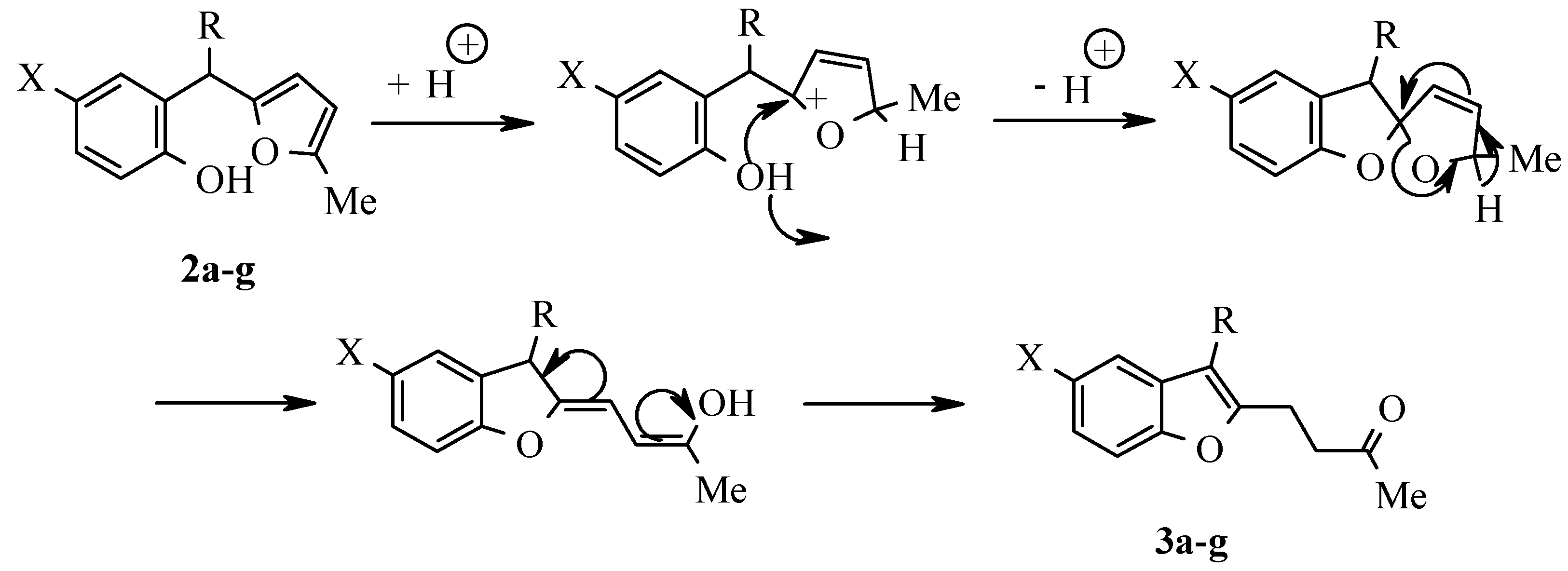
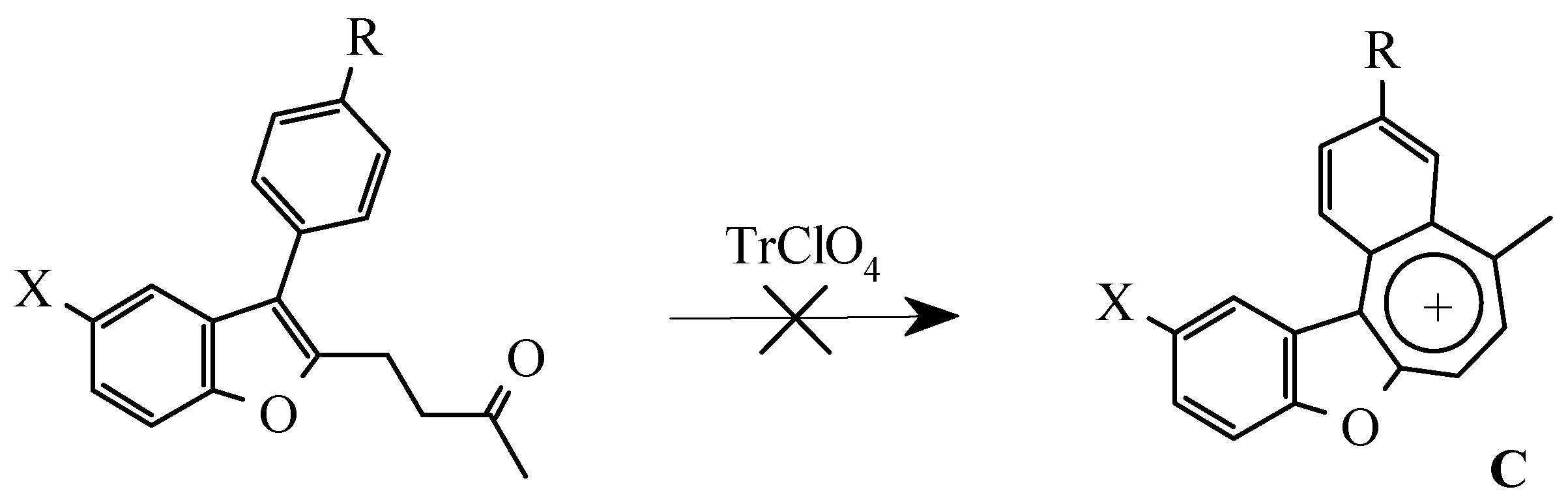
Results and Discussion

Experimental
General
General method of synthesis of 2-hydroxybenzyl alcohols 1
General method of synthesis of 2-hydroxyaryl-R - (5-methylfur-2-yl)methanes 2
General method of synthesis of 3-R-2 - (3-oxobutyl)benzo[b]furans 3
References and Notes
- Butin, A. V.; Stroganova, T. A.; Abaev, V. T.; Kul'nevich, V. G. Khim. Geterotsikl. Soedin. 1998, 1250.
- Gutnov, A. V.; Abaev, V. T.; Butin, A. V.; Zavodnik, V. E.; Kul'nevich, V. G. Khim. Geterotsikl. Soedin. 1996, 162.
- Abaev, V. T.; Gutnov, A. V.; Butin, A. V. Khim. Geterotsikl. Soedin. 1998, 603.
- Butin, A. V.; Abaev, V. T.; Zavodnik, V. E.; Kul'nevich, V. G. Khim. Geterotsikl. Soedin. 1993, 627.
- Butin, A. V.; Gutnov, A. V.; T. A. Abaev, V. T.; Krapivin, G. D. Khim. Geterotsikl. Soedin. 1998, 883.
- Butin, A. V.; Gutnov, A. V.; T. A. Abaev, V. T.; Krapivin, G. D. Molecules 1999, 52.
- Butin, A. V.; Krapivin, G. D.; Zavodnik, V. E.; Kul'nevich, V. G. Khim. Geterotsikl. Soedin. 1993, 616.
- Herbstein, F.N.; Schmidt, G.M.J. J. Chem. Soc. 1954, 3302.
- Dewar, M. J. S.; Ganellin, C.R. J.Chem. Soc. 1959, 3139.
- Berti, G.; Da Settimo, A. Ann. Chimica 1962, 995.
- Samples Availability: Available from MDPI.


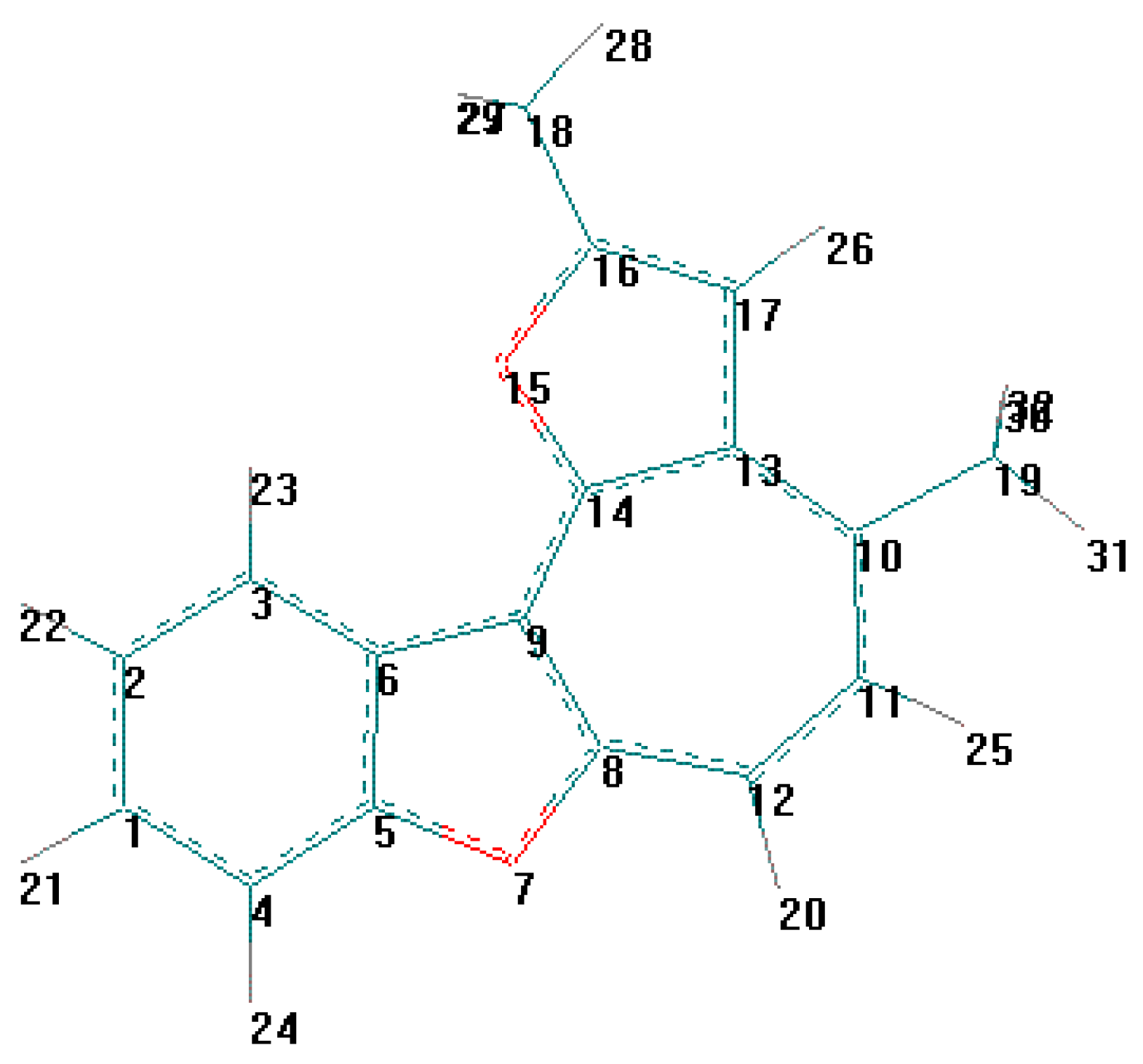
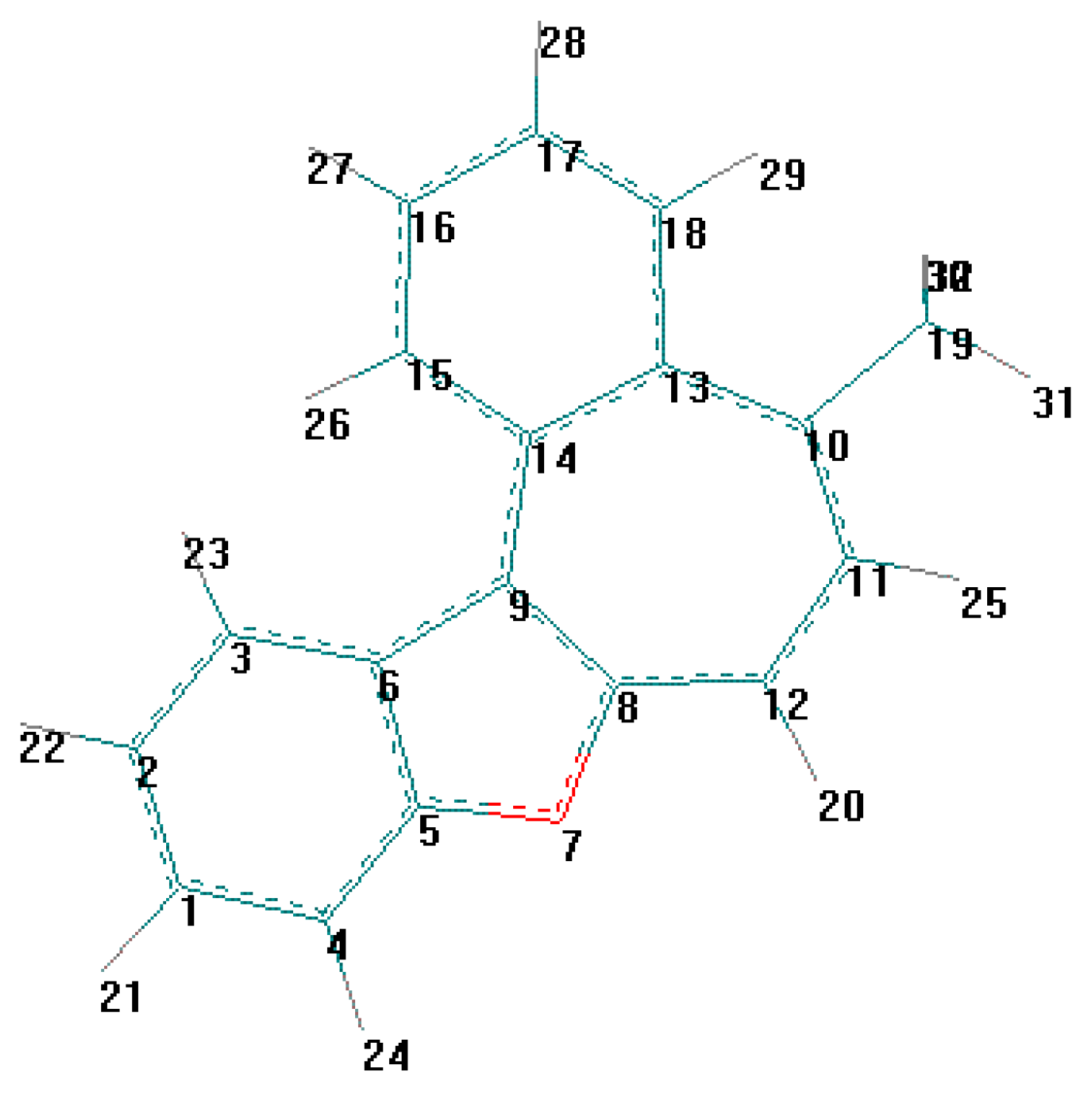
| Comp | R | X | Formula, Mr | Wi (calc.)% Wi (found)% | Yield% | IR-spectra cm-1 (bs, OH) | |
|---|---|---|---|---|---|---|---|
| C | H | ||||||
| 2a |  | CH3 | C19H18O2 | 82.08 | 6.58 | 99 | 3560 |
| 278.35 | 81.99 | 6.52 | |||||
| 2b |  | CH3 | C19H17BrO2 | 63.92 | 4.75 | 99 | 3540 |
| 357.25 | 63.88 | 4.80 | |||||
| 2c |  | CH3 | C20H20O2 | 82.08 | 6.86 | 98 | 3540 |
| 292.38 | 82.16 | 6.89 | |||||
| 2d |  | H | C18H16O2 | 81.77 | 6.16 | 98 | 3560 |
| 264.32 | 81.79 | 6.10 | |||||
| 2e |  | H | C19H18O2 | 82.05 | 6.54 | 99 | 3550 |
| 278.35 | 81.99 | 6.52 | |||||
| 2f |  | CH3 | C20H20O2 | 82.13 | 6.84 | 97 | 3530 |
| 292.38 | 82.16 | 6.89 | |||||
| 2g | -CH2CH3 | CH3 | C15H18O2 | 78.27 | 7.99 | 98 | 3440 |
| 230.31 | 78.23 | 7.88 | |||||
| Comp. | δ, ppm |
|---|---|
| 2a | 2.18 (s, 3H, CH3), 2.24 (s, 3H, CH3), 4.81 (bs, 1H, OH), 5.41 (s, 1H, CH), 5.67-5.87 (m, 2H, HFur), 6.62-7.34 (m, 8H, HAr) |
| 2b | 2.15 (s, 3H, CH3), 2.19 (s, 3H, CH3), 4.59 (bs, 1H, OH), 5.40 (s, 1H, CH), 5.65 (d, J = 3.2 Hz, 1H, 3-HFur), 5.77 (d, J = 3.2 Hz, 1H, 4-HFur), 6.42-7.53 (m, 7H, HAr) |
| 2c | 2.14 (s, 3H, CH3), 2.17 (s, 3H, CH3), 2.24 (s, 3H, CH3), 4.69 (bs, 1H, OH), 5.35 (s, 1H, CH), 5.62 (d, J = 3.2 Hz, 1H, 3-HFur), 5.74 (d, J = 3.2 Hz, 1H, 4-HFur), 6.48-7.19 (m, 7H, HAr) |
| 2d | 2.17 (s, 3H, CH3), 5.02 (bs, 1H, OH), 5.56 (s, 1H, CH), 5.73 (d, J = 3.2 Hz, 1H, 3-HFur), 5.81 (d, J = 3.2 Hz, 1H, 4-HFur), 6.60-7.53 (m, 9H, HAr) |
| 2e | 2.18 (s, 3H, CH3), 2.25 (s, 3H, CH3), 4.98 (bs, 1H, OH), 5.50 (s, 1H, CH), 5.47 (d, J = 3.2 Hz, 1H, 3-HFur), 5.82 (d, J = 3.2 Hz, 1H, 4-HFur), 6.64-7.32 (m, 8H, HAr) |
| 2f | 2.13 (s, 3H, CH3), 2.18 (s, 3H, CH3), 3.10 (d, J = 7.0 Hz, 1H, CH2), 3.18 (d, J = 7.0 Hz, 1H, CH2), 4.28 (t, J = 7.0 Hz, 1H, CH), 4.83 (bs, 1H, OH), 5.68 (d, J = 3.2 Hz, 1H, 4-HFur), 5.79 (d, J = 3.2 Hz, 1H, 3-HFur), 6.82-7.09 (m, 8H, HAr) |
| 2g | 0.83 (t, J=7.1 Hz, 3H, CH2CH3,), 1.23 (m, 2H, CH2CH3), 2.16 (s, 3H, CH3), 2.26 (s, 3H, CH3), 3.92 (d, JCH=7.5 Hz, 1H, CH), 4.96 (bs, 1H, OH), 5.76 (d, J =3.2 Hz, 1H, 3-HFur), 5.71 (d, J3,4Fur=3.2 Hz, 1H, 4-HFur), 6.37-7.13 (m, 3H, HAr) |
| Comp. | Formula, Mr | Wi (calc.)% Wi (found)% | Yield% | M.p.(°C) | |
|---|---|---|---|---|---|
| C | H | ||||
| 3a | C19H18O2 | 81.95 | 6.55 | 92 | 57-58 |
| 278.35 | 81.99 | 6.52 | |||
| 3b | C19H17BrO2 | 63.92 | 4.77 | 91 | 81-82 |
| 357.25 | 63.88 | 4.80 | |||
| 3c | C20H20O2 | 82.19 | 6.86 | 95 | oil |
| 292.38 | 82.16 | 6.89 | |||
| 3d | C18H16O2 | 81.84 | 6.18 | 95 | 39-40 |
| 264.32 | 81.79 | 6.10 | |||
| 3e | C19H18O2 | 82.05 | 6.55 | 93 | 68-69 |
| 278.35 | 81.99 | 6.52 | |||
| 3f | C20H20O2 | 82.21 | 6.81 | 90 | 41-42 |
| 292.38 | 82.16 | 6.89 | |||
| 3g | C15H18O2 | 78.12 | 7.80 | 90 | oil |
| 230.31 | 78.23 | 7.88 | |||
| Comp | δ, ppm |
|---|---|
| 3a | 2.09 (s, 3H, CH3CO), 2.35 (s, 3H, CH3), 2.68-2.93 (m, 2H, β-CH2), 2.96-3.21 (m, 2H, α-CH2), 6.91-7.49 (m, 8H, HBf + HAr) |
| 3b | 2.08 (s, 3H, CH3CO), 2.34 (s, 3H, CH3), 2.67-2.91 (m, 2H, β-CH2), 2.92-3.16 (m, 2H, α-CH2), 6.98 (d, J = 8.1 Hz, 1H, 6-HBf), 7.20 (d, 1H, 4-HBf), 7.24 (d, J = 8.1 Hz, 1H, 7-HBf), 7.27 (d, J = 8.8 Hz, 2H, m-HAr), 7.52 (d, J = 8.8 Hz, 2H, o-HAr) |
| 3c | 2.08 (s, 3H, CH3CO), 2.34 (s, 6H, CH3), 2.67-2.92 (m, 2H, β-CH2), 2.95-3.20 (m, 2H, α-CH2), 6.90-7.40 (m, 7H, HBf + HAr) |
| 3d | 2.07 (s, 3H, CH3CO), 2.69-2.94 (m, 2H, β-CH2), 2.97-3.22 (m, 2H, α-CH2), 7.09-7.57 (m, 9H, HBf + HAr) |
| 3e | 2.08 (s, 3H, CH3CO), 2.34 (s, 3H, CH3), 2.69-2.94 (m, 2H, β-CH2), 2.97-3.22 (m, 2H, α-CH2), 7.07-7.56 (m, 8H, HBf + HAr) |
| 3f | 2.06 (s, 3H, CH3CO), 2.28 (s, 3H, CH3), 2.62-2.83 (m, 2H, β-CH2), 2.86-3.07 (m, 2H, α-CH2), 3.91 (s, 2H, CH2), 7.07-7.56 (m, 8H, HBf + HAr) |
| 3g | 1.16 (t, J = 7.7 Hz, 3H, CH2CH3) 2.10 (s, 3H, CH3CO), 2.37 (s, 3H, CH3), 2.56 (k, J = 7.7 Hz, 2H, CH2CH3) 2.60-2.85 (m, 2H, β-CH2), 2.87-3.02 (m, 2H, α-CH2), 6.94 (d, J = 8.1 Hz, 1H, 6-HBf), 7.16 (d, J = 8.1 Hz, 1H, 7-HBf) 7.18 (d, 1H, 4-HBf) |
| Unit contents: | C(19)H(18)O(2) | |
| Space group: | P2(1)/c, Z = 4 | |
| Crystal system: | monoclinic | |
| Unit cell dimensions: | a = 5.917(1)A | Alpha = 90 deg. |
| b = 25.393(2)A | Beta = 101.08 (1) deg. | |
| c = 10.281(1)A | Gamma = 90 deg. | |
| Volume: | 1515.9 (.6) A3 | |
| Density (calculated): | 1.220 g/cm3 | |
| Diffractometer: | Syntex P1 | |
| Scan technique: | Theta/2*Theta | |
| Monochromator: | none | |
| Wave length: | .73 cm-1 | |
| sin(Th)/WL(max): | .6168 | |
| Lambda: | Mo (K-alpha) | |
| Number of reflections: | 1898 I > 3.00*s (I) | |
| Programs: | SHELXTL | |
| Solution: | Direct method | |
| Final R factors: | R = .044 Rw = .050 | |
| GOOF: | 2.10 | |
| Weight: | 1/(sigma** (F) + .000439*F*F) |
| Atom | x | Y | z | Ueq |
| O(1) | 2750 (2) | 5797 (1) | 4448 (1) | 47 (1) * |
| O(2) | 3568 (3) | 4516 (1) | 8262 (2) | 82 (1) * |
| C(1) | 650 (3) | 6537 (1) | 4561 (2) | 39 (1) * |
| C(2) | -128 (4) | 7038 (1) | 4135 (2) | 48 (1) * |
| C(3) | 593 (5) | 7253 (1) | 3055 (2) | 58 (1) * |
| C(4) | 2105 (5) | 6985 (1) | 2408 (3) | 59 (1) * |
| C(5) | 2914 (5) | 6490 (1) | 2817 (2) | 53 (1) * |
| C(6) | 2145 (4) | 6279 (1) | 3873 (2) | 42 (1) * |
| C(7) | 1668 (4) | 5760 (1) | 5520 (2) | 40 (1) * |
| C(8) | 387 (3) | 6190 (1) | 5646 (2) | 37 (1) * |
| C(9) | -892 (4) | 6314 (1) | 6703 (2) | 37 (1) * |
| C(10) | 7 (4) | 6208 (1) | 8029 (2) | 45 (1) * |
| C(11) | -1124 (4) | 6350 (1) | 9019 (2) | 49 (1) * |
| C(12) | -3226 (4) | 6615 (1) | 8741 (2) | 48 (1) * |
| C(13) | -4144 (4) | 6718 (1) | 7423 (2) | 46 (1) * |
| C(14) | -3021 (4) | 6571 (1) | 6421 (2) | 42 (1) * |
| C(15) | -4417 (6) | 6784 (2) | 9839 (3) | 68 (1) * |
| C(16) | 2187 (4) | 5266 (1) | 6310 (2) | 47 (1) * |
| C(17) | 4624 (4) | 5254 (1) | 7116 (3) | 48 (1) * |
| C(18) | 5065 (4) | 4816 (1) | 8105 (2) | 51 (1) * |
| C(19) | 7478 (6) | 4776 (1) | 8881 (4) | 73 (1) * |
| O(1) - C(6) | 1.376 (2) | O(1) - C(7) | 1.380 (3) |
| O(2) - C(18) | 1.203 (3) | C(1) - C(2) | 1.395 (3) |
| C(1) - C(6) | 1.396 (3) | C(1) - C(8) | 1.452 (3) |
| C(2) - C(3) | 1.376 (4) | C(3) - C(4) | 1.391 (4) |
| C(4) - C(5) | 1.383 (3) | C(5) - C(6) | 1.365 (3) |
| C(7) - C(8) | 1.349 (3) | C(7) - C(16) | 1.495 (3) |
| C(8) - C(9) | 1.473 (3) | C(9) - C(10) | 1.391 (3) |
| C(9) - C(14) | 1.399 (3) | C(10) - C(11) | 1.370 (3) |
| C(11) - C(12) | 1.395 (3) | C(12) - C(13) | 1.384 (3) |
| C(12) - C(15) | 1.504 (4) | C(13) - C(14) | 1.380 (3) |
| C(16) - C(17) | 1.519 (3) | C(17) - C(18) | 1.495 (3) |
| C(18) - C(19) | 1.499 (4) | ||
| C(6) - O(1) - C(7) | 106.3 (2) | C(2) - C(1) - C(6) | 118.1 (2) |
| C(2) - C(1) - C(8) | 135.7 (2) | C(6) - C(1) - C(8) | 106.1 (2) |
| C(1) - C(2) - C(3) | 118.7 (2) | C(2) - C(3) - C(4) | 121.3 (2) |
| C(3) - C(4) - C(5) | 121.0 (2) | C(4) - C(5) - C(6) | 116.8 (2) |
| O(1) - C(6) - C(1) | 109.7 (2) | O(1) - C(6) - C(5) | 126.2 (2) |
| C(1) - C(6) - C(5) | 124.0 (2) | O(1) - C(7) - C(8) | 112.2 (2) |
| O(1) - C(7) - C(16) | 114.4 (2) | C(8) - C(7) - C(16) | 133.5 (2) |
| C(1) - C(8) - C(7) | 105.7 (2) | C(1) - C(8) - C(9) | 125.7 (2) |
| C(7) - C(8) - C(9) | 128.4 (2) | C(8) - C(9) - C(10) | 121.9 (2) |
| C(8) - C(9) - C(14) | 121.1 (2) | C(10) - C(9) - C(14) | 117.0 (2) |
| C(9) - C(10) - C(11) | 121.8 (2) | C(10) - C(11) - C(12) | 121.2 (2) |
| C(11) - C(12) - C(13) | 117.3 (2) | C(11) - C(12) - C(15) | 120.8 (2) |
| C(13) - C(12) - C(15) | 121.9 (2) | C(12) - C(13) - C(14) | 121.7 (2) |
| C(9) - C(14) - C(13) | 121.0 (2) | C(7) - C(16) - C(17) | 112.8 (2) |
| C(16) - C(17) - C(18) | 114.2 (2) | O(2) - C(18) - C(17) | 121.7 (2) |
| O(2) - C(18) - C(19) | 122.4 (2) | C(17) - C(18) - C(19) | 115.9 (2) |
| C(1) - C(2) | 1.400 (5) | 1.403 | C(2) - C(3) | 1.368 (5) | 1.394 |
| C(3) - C(6) | 1.412 (5) | 1.394 | C(5) - C(6) | 1.391 (5) | 1.430 |
| C(4) - C(5) | 1.387 (5) | 1.387 | C(1) - C(4) | 1.387 (5) | 1.398 |
| C(5) - O(7) | 1.374 (4) | 1.404 | O(7) - C(8) | 1.375 (4) | 1.401 |
| C(8) - C(9) | 1.411 (5) | 1.434 | C(6) - C(9) | 1.446 (5) | 1.448 |
| C(8) - C(12) | 1.382 (5) | 1.390 | C(11) - C(12) | 1.385 (5) | 1.385 |
| C(10) - C(11) | 1.399 (5) | 1.399 | C(10) - C(13) | 1.411 (5) | 1.397 |
| C(13) - C(14) | 1.406 (5) | 1.436 | C(9) - C(14) | 1.377 (5) | 1.383 |
| C(10) - C(19) | 1.513 (5) | 1.490 | O(15) - C(14) | 1.358 (4) | 1.394 |
| O(15) - C(16) | 1.382 (4) | 1.410 | C(16) - C(17) | 1.339 (5) | 1.377 |
| C(13) - C(17) | 1.430 (5) | 1.455 | C(16) - C(18) | 1.481 (5) | 1.463 |
| C(1) - C(2) - C(3) | 122.3 (3) | 121.7 | C(2) - C(3) - C(6) | 116.9 (3) | 118.0 |
| C(3) - C(6) - C(5) | 119.9 (3) | 119.2 | C(4) - C(5) - C(6) | 123.5 (3) | 123.3 |
| C(1) - C(4) - C(5) | 115.6 (3) | 116.0 | C(2) - C(1) - C(4) | 121.8 (3) | 121.8 |
| C(5) - O(7) - C(8) | 106.9 (3) | 106.4 | O(7) - C(8) - C(9) | 110.1 (3) | 110.4 |
| C(6) - C(9) - C(8) | 106.0 (3) | 106.4 | C(5) - C(6) - C(9) | 105.6 (3) | 106.0 |
| C(6) - C(5) - O(7) | 111.5 (3) | 110.8 | C(8) - C(12) - C(11) | 127.5 (3) | 127.2 |
| C(10) - C(11) - C(12) | 131.2 (3) | 130.8 | C(11) - C(10) - C(13) | 125.4 (3) | 126.3 |
| C(10) - C(13) - C(14) | 127.9 (3) | 129.1 | C(9) - C(14) - C(13) | 132.8 (3) | 130.9 |
| C(8) - C(9) - C(14) | 123.7 (3) | 123.6 | C(9) - C(8) - C(12) | 131.4 (3) | 132.1 |
| C(11) - C(10) - C(19) | 116.8 (3) | 117.1 | C(14) - O(15) - C(16) | 107.0 (3) | 107.1 |
| O(15) - C(16) - C(17) | 110.6 (3) | 110.4 | C(13) - C(17) - C(16) | 107.7 (3) | 107.7 |
| C(14) - C(13) - C(17) | 105.0 (3) | 105.4 | C(13) - C(14) - O(15) | 109.7 (3) | 109.5 |
| C(1) - C(2) | 1.403 | C(2) - C(3) | 1.391 |
| C(3) - C(6) | 1.404 | C(5) - C(6) | 1.435 |
| C(4) - C(5) | 1.391 | C(1) - C(4) | 1.391 |
| C(5) - O(7) | 1.389 | O(7) - C(8) | 1.406 |
| C(8) - C(9) | 1.429 | C(6) - C(9) | 1.466 |
| C(8) - C(12) | 1.384 | C(11) - C(12) | 1.380 |
| C(10) - C(11) | 1.389 | C(10) - C(13) | 1.431 |
| C(13) - C(14) | 1.438 | C(9) - C(14) | 1.425 |
| C(10) - C(19) | 1.499 | C(14) - C(15) | 1.429 |
| C(15) - C(16) | 1.372 | C(16) - C(17) | 1.407 |
| C(17) - C(18) | 1.372 | C(13) - C(18) | 1.436 |
| C(3) - H(23) | 1.098 | C(15) - H(26) | 1.095 |
| H(23) … H(26) | 1.628 |
| C(1) - C(2) - C(3) | 122.4 | C(2) - C(3) - C(6) | 119.9 |
| C(3) - C(6) - C(5) | 115.5 | C(4) - C(5) - C(6) | 125.6 |
| C(1) - C(4) - C(5) | 116.3 | C(2) - C(1) - C(4) | 120.4 |
| C(5) - O(7) - C(8) | 106.0 | O(7) - C(8) - C(9) | 111.7 |
| C(6) - C(9) - C(8) | 104.9 | C(5) - C(6) - C(9) | 106.1 |
| C(6) - C(5) - O(7) | 111.2 | C(8) - C(12) - C(11) | 125.6 |
| C(10) - C(11) - C(12) | 129.9 | C(11) - C(10) - C(13) | 129.2 |
| C(10) - C(13) - C(14) | 128.6 | C(9) - C(14) - C(13) | 127.0 |
| C(8) - C(9) - C(14) | 125.2 | C(9) - C(8) - C(12) | 134.4 |
| C(11) - C(10) - C(19) | 112.7 | C(14) - C(15) - C(16) | 124.1 |
| C(15) - C(16) - C(17) | 119.0 | C(16) - C(17) - C(18) | 119.0 |
| C(13) - C(18) - C(17) | 123.9 | C(13) - C(14) - C(15) | 117.0 |
| C(14) - C(15) - H(26) | 119.2 | C(6) - C(3) - H(23) | 123.8 |
© 1999 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gutnov, A.V.; Butin, A.V.; Abaev, V.T.; Krapivin, G.D.; Zavodnik, V.E. Furyl(aryl)alkanes and Their Derivatives. 19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring Closure Type. Molecules 1999, 4, 204-218. https://doi.org/10.3390/40700204
Gutnov AV, Butin AV, Abaev VT, Krapivin GD, Zavodnik VE. Furyl(aryl)alkanes and Their Derivatives. 19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring Closure Type. Molecules. 1999; 4(7):204-218. https://doi.org/10.3390/40700204
Chicago/Turabian StyleGutnov, Andrey V., Alexander V. Butin, Vladimir T. Abaev, Gennadij D. Krapivin, and Valerij E. Zavodnik. 1999. "Furyl(aryl)alkanes and Their Derivatives. 19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring Closure Type" Molecules 4, no. 7: 204-218. https://doi.org/10.3390/40700204




