Synthesis of Meldrum's acids 1a-e
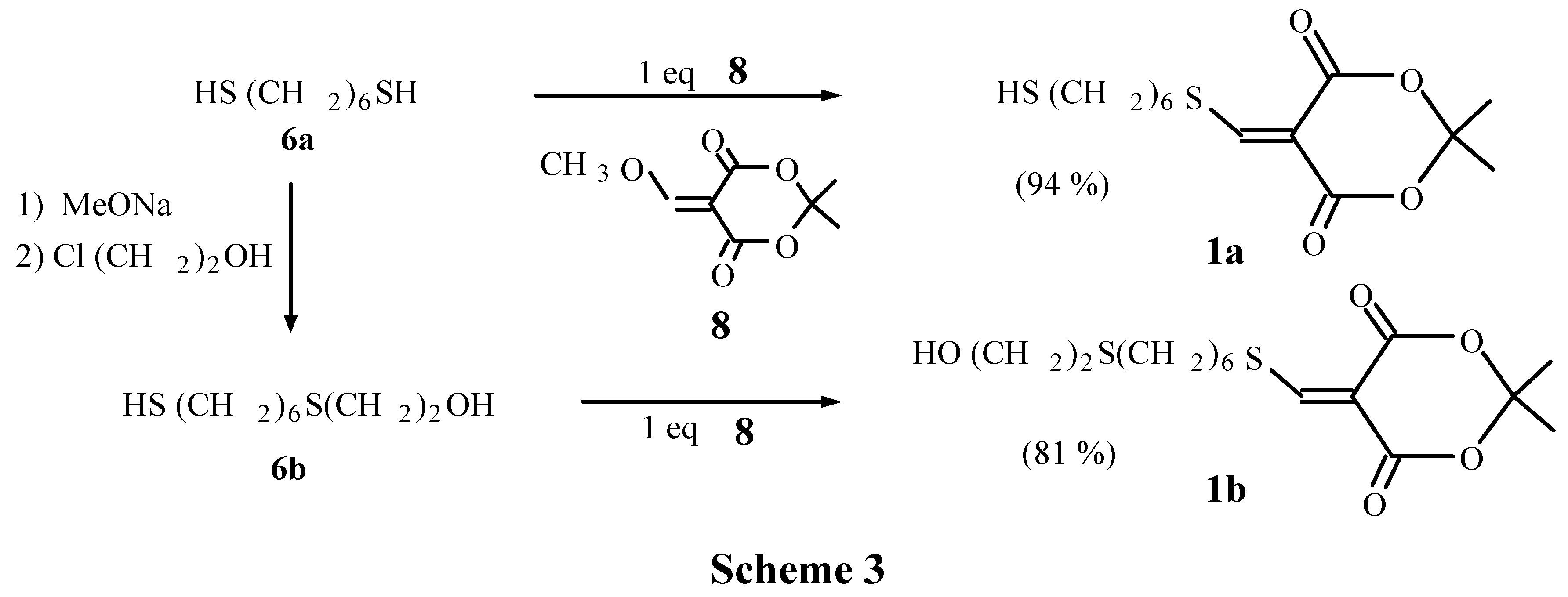
6-Mercapto hexylsulfanyl methylene Meldrum's acid (1a)
Enol ether
8 [
3] (0.62 g, 33x10
-4 mol) and 1,6 hexanedithiol (0.5 g, 33x10
-4 mol) in acetonitrile (50mL) were placed in a two necked flask equipped with a reflux condenser. The solution was refluxed for 12-15 h and the solvent was then evaporated under vacuum. The crude product was purified by chromatography on silica gel (elution with 1:1 CH
2Cl
2/petroleum ether) to give 0.95 g (31x10
-4 mol, 94%) of a white solid. Mp = 160°C.
1H-NMR: δ = 1.25 -1.50 (m, 5H); 1.55 (m, 2H); 1.65 (s, 6H); 1.75 (m, 2H); 2.45 (m, 2H); 2.95 (t, 2H, J =7.1Hz); 8.95 (s, 1H). MS m/z (%): 304 (M
+., 3), 247 (8), 246 (31) , 203 (16), 202 (4), 150 (31), 148 (27), 126 (34), 117 (12), 116 (40), 87 (91), 69 (37), 67 (59), 60 (48), 57 (59), 55 (94), 443 (100). IR (KBr): 1716(ν
C=O), 1520(ν
C=C).
9-Hydroxy-7-thianonylsulfanyl methylene Meldrum's acid (1b)
1b was prepared by a similar procedure from enol ether 8 (0.48 g, 26.10-4 mol) and 9-mercapto-2-thianonanol (0.5 g, 26x10-4 mol). The purification was performed by chromatography with acetone/ methanol: (10:1); 0.73 g of 1b was obtained (81%). 1H-NMR: δ = 1.39 (m, 4H); 1.55 (m, 2H); 1.67 (s, 6 H); 1.72 (m, 2H); 2.2 (s, 1H); 2.47 (t, 2H, J =7.3Hz); 2.65 (m, 2H); 2.95 (t, 2H, J =7.3Hz); 3.66 (t, 2H, J = 6.0Hz); 8.94 (s, 1H). MS m/z (%): 348 (M+., 2), 246 (3), 161 (40), 86 (100), 84 (49), 61 (85), 55 (76), 43 (95), 41 (63). IR (KBr): 1712 (νC=O), 1520 (νC=C).
Hexamethylenedithio-bis-methylene Meldrum's acid (1c)
1c was prepared by a similar procedure from enol ether 8 (2.48 g, 132x10-4 mol) and hexanedithiol (1 g, 66x10-4 mol). Purification was performed by chromatography using chloroform/acetone (2:1); 2.65 g of 1c was obtained (87%). Mp = 162°C. 1H-NMR: δ = 1.5 (m, 4H); 1.73 (s, 12H); 1.8 (m, 4H); 3.02 (t, 4H, J =7.3Hz); 8.98 (s, 2H). MS m/z (%): 458 (M+., 2), 400 (5), 296 (22), 246 (49), 241 (29), 165 (20), 139 (36), 1321 (19), 43 (100). IR (KBr): 1716 (νC=O), 1518 (νC=C).
N-Methylpropargylaminomethylene Meldrum's acid (1d)
1d was prepared by a similar procedure from enol ether 8 (6.7 g, 36x10-3 mol) and N-methylpropargylamine (2.5 g, 36x10-3 mol). Recrystallisation of the crude mixture from ethanol gave 5.6 g (25x10-3 mol, 70%) of 1d. Mp = 123°C. 1H-NMR showed two isomers: δ = 1.72 (s, 6H); 2.43 (t, 1H, J = 3.0Hz); 3.52 (s, 3H); 4.77 (d, 2H, J = 3.0Hz); 8.10 (s, 1H); and 1.73 (s, 6H); 2.65 (t, 1H, J = 3.0Hz); 3.42 (s, 3H); 4.31 (d, 2H, J = 3.0Hz), 8.32 (s, 1H). MS m/z (%): 223 (M+., 5), 208 (17), 166 (50), 165 (19), 122 (100), 121 (33), 93 (30), 82 (53), 68 (10), 67 (17), 66 (35), 65 (18). IR (CHCl3): 3280 (ν H-C+), 2100 (νC+C), 1680 (νC=O), 1590 (νC=C).
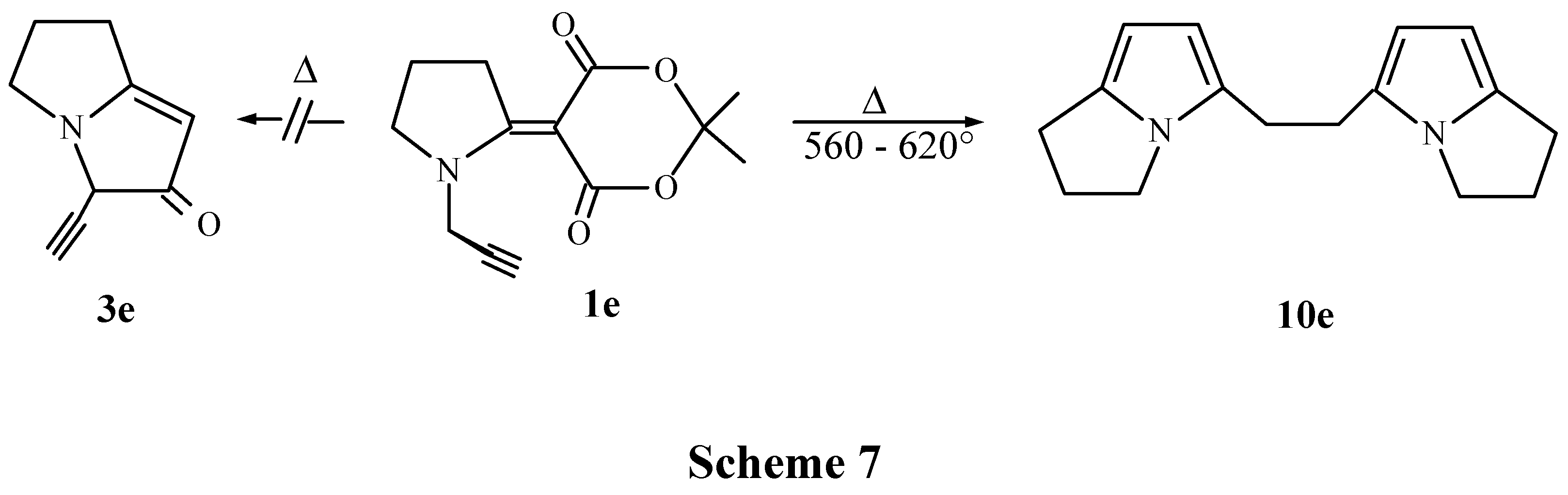
1-(Propargyl)-2-pyrrolidinylmethylene Meldrum's acid (1e)
1e was prepared from N-propargylpyrrolidin-2-one following the procedure previously reported [
6,
11]
1H-NMR: δ = 1.76 (s, 6H); 2.15 (qt, 2H, J =8.0Hz); 2.42 (t, 1H, J =3.0Hz); 3.53 (t, 2H, J = 8.0Hz); 3.97 (t, 2H, J = 8.0Hz); 4.45 (d, 2H, J = 3.0Hz). MS m/z (%): 249 (M
+., 1) , 234 (12), 192 (20), 149(10), 148(95), 147(9), 119(38), 86(100). IR (CHCl
3): 3300 (ν
H-C≡), 2130 (ν
C≡C), 1710 (ν
C=O) , 1550 (ν
C=C).
Thermolysis of Meldrum's acids 1a-c
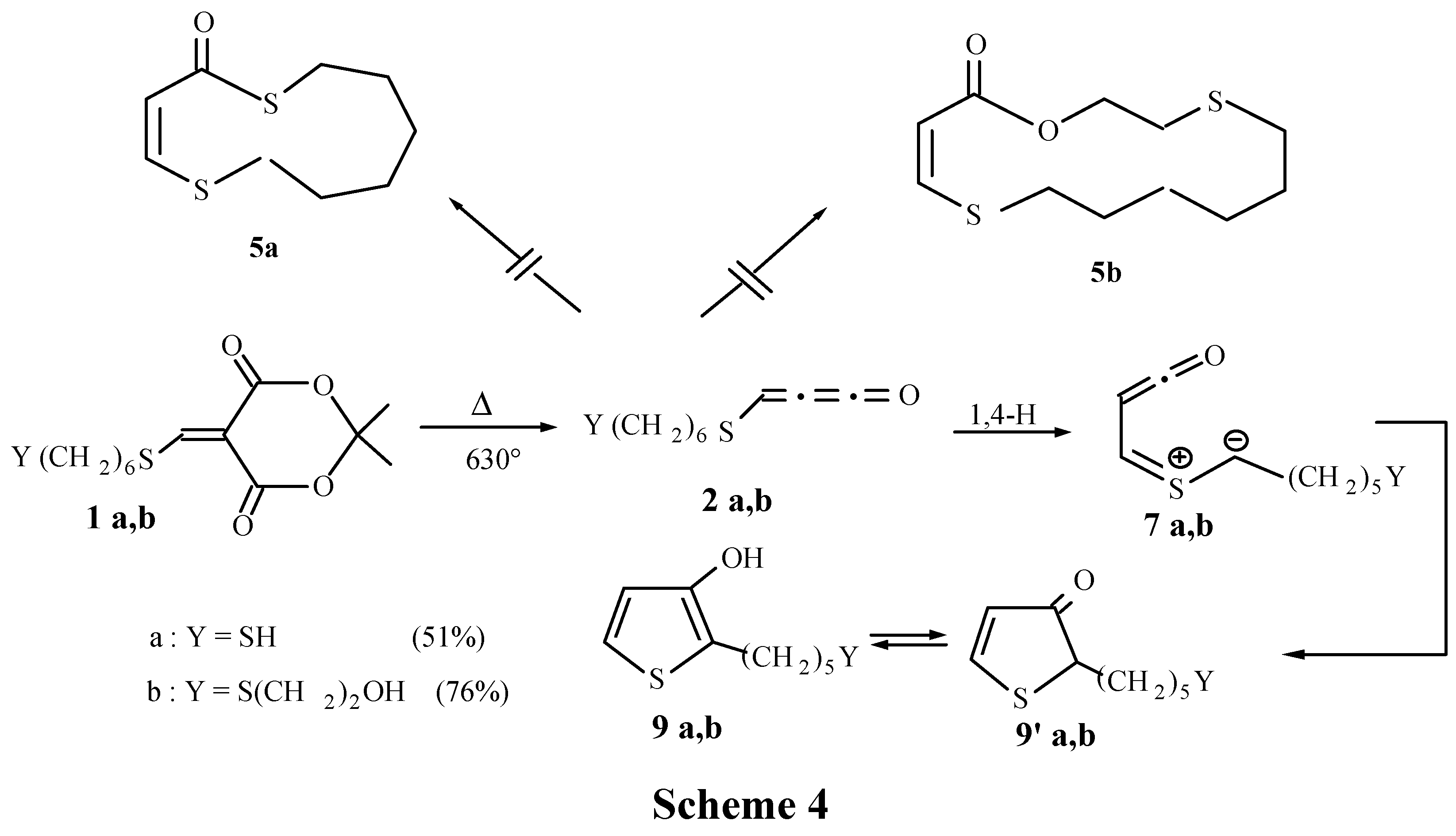
Thermolysis of derivatives 1a,c were carried out under flow conditions. Solutions of 1 were vaporised at the top of an electrical heated quartz column filled with quartz beads. The temperature was held constant during the thermolysis; the pressure was maintained at 5x10-2 Torr. Products were condensed at -196°C and analysed after purification.
Thermolysis of 1a: T=630°C; 9a and 9'a were purified by chromatography on silica gel with CH2Cl2,/acetone (66/34). Yield: 68 mg (3.36x10-4 mol, 51%) from 200 mg (6.58x10-4 mol) of 1a. 1H-NMR of 9a: δ = 1.3 -1.6 (m, 9H); 2.65 (m, 3H); 6.56 (d, 1H, J = 5.4Hz); 6.85 (d, 1H, J = 5.4Hz); 1H-NMR of 9'a: δ = 1.3 -1.6 (m, 9H); 2.65 (m, 2H); 3.60 (m, 1H); 6.12 (d, 1H, J = 5.7Hz); 8.28 (d, 1H, J = 5.7Hz). MS m/z (%) : 202 (M+., 14), 139 (32), 113 (100), 87 (32), 55 (20).
Thermolysis of 1b: T=540°C; 9b and 9'b were purified by chromatography on silica gel with acetone. Yield: 107 mg (4.35x10-4 mol, 76%) from 200 mg (5.74x10-4 mol) of 1b.1H-NMR of 9b: δ =1.36 (m, 4 H); 1.52 (m, 5H); 2.47 (t, 2H, J =6.0 Hz); 2.65 (m, 3H); 3.65 (t, 2H, J = 6.0Hz); 6.55 (d, 1H, J = 5.6Hz); 6.85 (d, 1H, J = 5.6Hz); 1H-NMR of 9'b showed two specific signals for this tautomer: 6.13 (d, 1H, J = 5.7Hz); 8.3 (d, 1H, J = 5.7Hz). MS m/z (%): 246 (M+., 3), 159 (15), 55 (100), 45 (30), 43 (51).
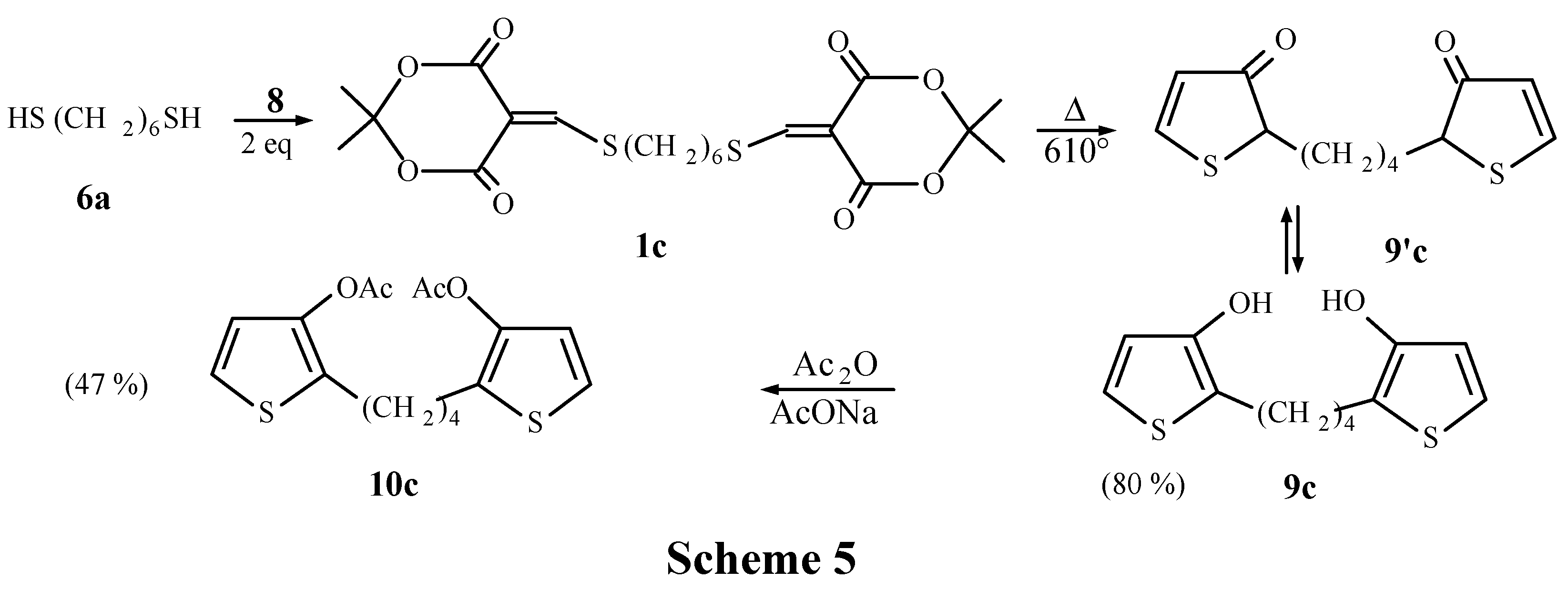
Thermolysis of 1c: 610°C; 9c and 9'c purified by chromatography with CH2Cl2. Yield: 80%. 1H-NMR of 9c (CDCl3): δ = 1.6-1.7 (m, 6H); 2.65 (m, 4H); 6.55 (d, 2H, J = 5.4Hz); 6.86 (d, 2H, J = 5.4Hz); 1H-NMR of 9'c: δ = 1.66 (m, 4H); 2.65 (m, 4H); 3.6 (m, 2H); 6.11 (d, 2H, J = 6.0Hz); 8.28 (d, 2H, J = 6.0Hz). 10c, purified by chromatography with CH2Cl2/acetone (90:10). Yield: 80 mg (2.36x10-4 mol, 47%) from 230 mg (5x10-4 mol) of 1c. 1H-NMR: δ = 1.60 (m, 4H); 2.19 (s, 6H); 2.60 (m, 4H); 6.73 (d, 2H, J = 5.5Hz); 6.98 (d, 2H, J = 5.5Hz); 13C-NMR: δ = 20.8(q); 25.7(t); 30.1(t); 120.8(d); 121.9(d); 129.7(s); 142.9(s); 169.1(s). MS m/z (%): 340 (3), 338 (3), 336 (6), 294 (28), 293 (100), 292 (48), 140 (25), 139 (75), 136 (28), 43 (34).
Thermolysis of Meldrum's Acids 1d,e
Pyrolyses of
1d,e were carried out at low pressures (10
-4-10
-5 Torr) in a apparatus previously described [
6,
12] and the products were collected on a cold finger, cooled in liquid N
2 at the exit of the horizontal quartz tube heated by an electrical oven. An entrance port placed between the exit of the tube and the cold finger permits the coating of the cold finger with methanol.
Bis 2'-(N-methylpyrrolyl) -1,2 -ethane (10d).
Purified by chromatography on silica gel with ether/petroleum ether/methanol (50:40:10). Yield of 10d: 146 mg (7.76x10-4 mol, 42%) from 819 mg (36.7x10-4 mol) of 1d. Mp = 97°C. 1H-NMR: δ =2.96 (s, CΗ2); 3.55 (s, CH3); 6.06 (d, 1H, J = 3.5Hz); 6.18 (d, 1H, J = 3.5Hz); 6.65 (s, 1H); 13C-NMR: δ = 26.3 (t) ; 33.5 (q) ; 105.7 (d) ; 106.8 (d) ; 121.3 (d); 132.8 (s); MS m/z (%): 189 (M+1, 17), 188 (M+., 98), 95 (67), 94 (100); IR (CHCl3): ν = 3010, 2960, 2950, 1500, 1455, 1420, 1305, 1090; Anal. Found: C, 76.49; H, 8.71; N, 14.75. Calc. for C12H16N2: C, 76.55; H, 8.56; N, 14.88%).
Bis 5'-(2,3-dihydro-1H-pyrrolizinyl) -1,2 -ethane (10e).
Purified by chromatography on silica gel with petroleum ether/ether (60:40). Yield of 10e: 123 mg (5.12x10-4 mol, 34%) from 760 mg (30.5x10-4 mol) of 1e. 1H-NMR: δ = 2.47 (qt, 2H, J = 7.1Hz); 2.85 (s, CH2); 2.87 (t, 2H, J = 7.1Hz); 3.68 (t, 2H, J = 7.1Hz); 5,77 (sl, 1H); 6.0 (sl, 1H); 13C-NMR: δ = 24.5 (t); 27.6 (t); 28.1 (t); 44.3 (t); 98;2 (d); 108.5 (d); 127.4 (s); 135.5 (s); MS m/z (%) =240 (M+., 10); 121 (12); 120 (100)