Results and Discussion
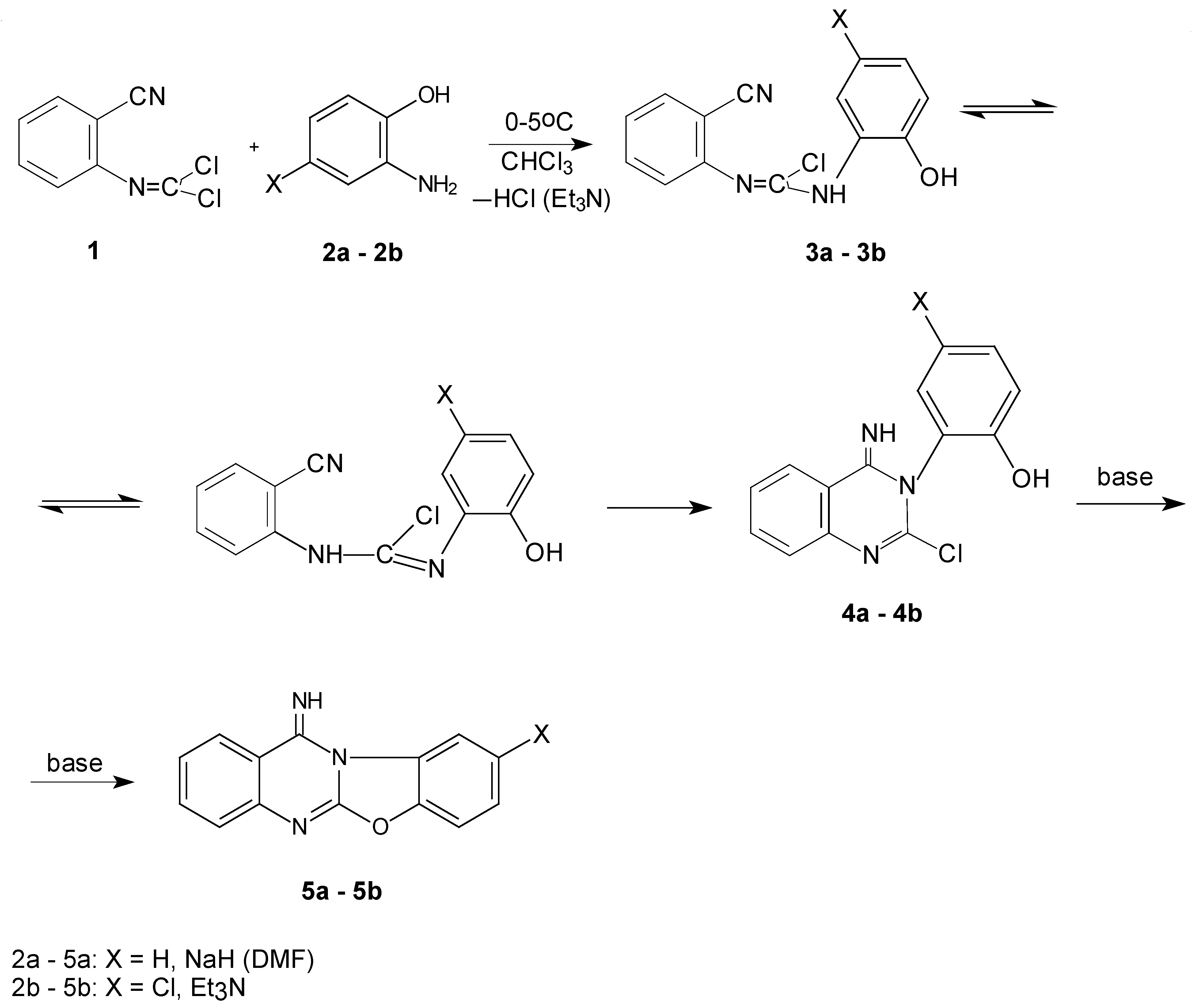
Our goal was to prepare 1,3-oxazolo- and 1,3-oxazino[2,3-b]quinazoline syntheses by a one–pot reaction of N-(2-cyanophenyl)chloromethanimidoyl chloride (1) with the aforementioned N,O-bifunctional nucleophiles. The starting compound 1 can act as a trifunctional electrophilic reagent – it can be attacked by two nucleophilic reagents on the carbon atom of the chloromethanimidoyl chloride group or on the carbon of the cyano group. We expected that attack of the N,O-bifunctional nucleophiles would be initiated on the primary amino group, which is more nucleophilic than the hydroxy group.
First we studied the room temperature reaction of
1 with 2-aminophenol
2a in the presence of one equivalent of triethylamine as a base (
Scheme 1) because the phenolic hydroxyl is less nucleophilic than the alcoholic one, we expected that consecutive attacks would be suppressed under those conditions and we would be able to investigate the intermediate(s) of this reaction. After the reaction was finished 2-chloro-3-(2-hydroxyphenyl)-3,4-dihydroquinazolin-4-imine (
4a) was separated and identified as the main product.
Since we did not obtain an acyclic intermediate
3a in the studied reaction we carried out the same reaction at a temperature of 0° to -5°C. Thus we successfully obtained
N-(2-cyanophenyl)-(2-hydroxy-anilino)methanimidoyl chloride (
3a) in this case. The identity of both intermediates
3a,
4a was supported by the elementary analysis, FTIR,
1H- (both tautomers of
3a) and
13C-NMR spectroscopy and in the case of
4a, by mass spectroscopy (
Scheme 2).
In the next step of the synthesis we attempted to cyclize
4a to the fused quinazoline
5a by heating in a chloroform solution in the presence of the second equivalent of triethylamine but the reaction was unsuccessful. The resulting
5a was obtained only by using sodium hydride as a base in dimethyl-formamide solution at room temperature. On the other hand, the analogous compound
5b was prepared in a
one-pot synthesis using 2 equivalents of triethylamine in chloroform solution. This finding may be explained by a higher acidity of the phenolic proton of the intermediate
4b than
4a (
Scheme 1)
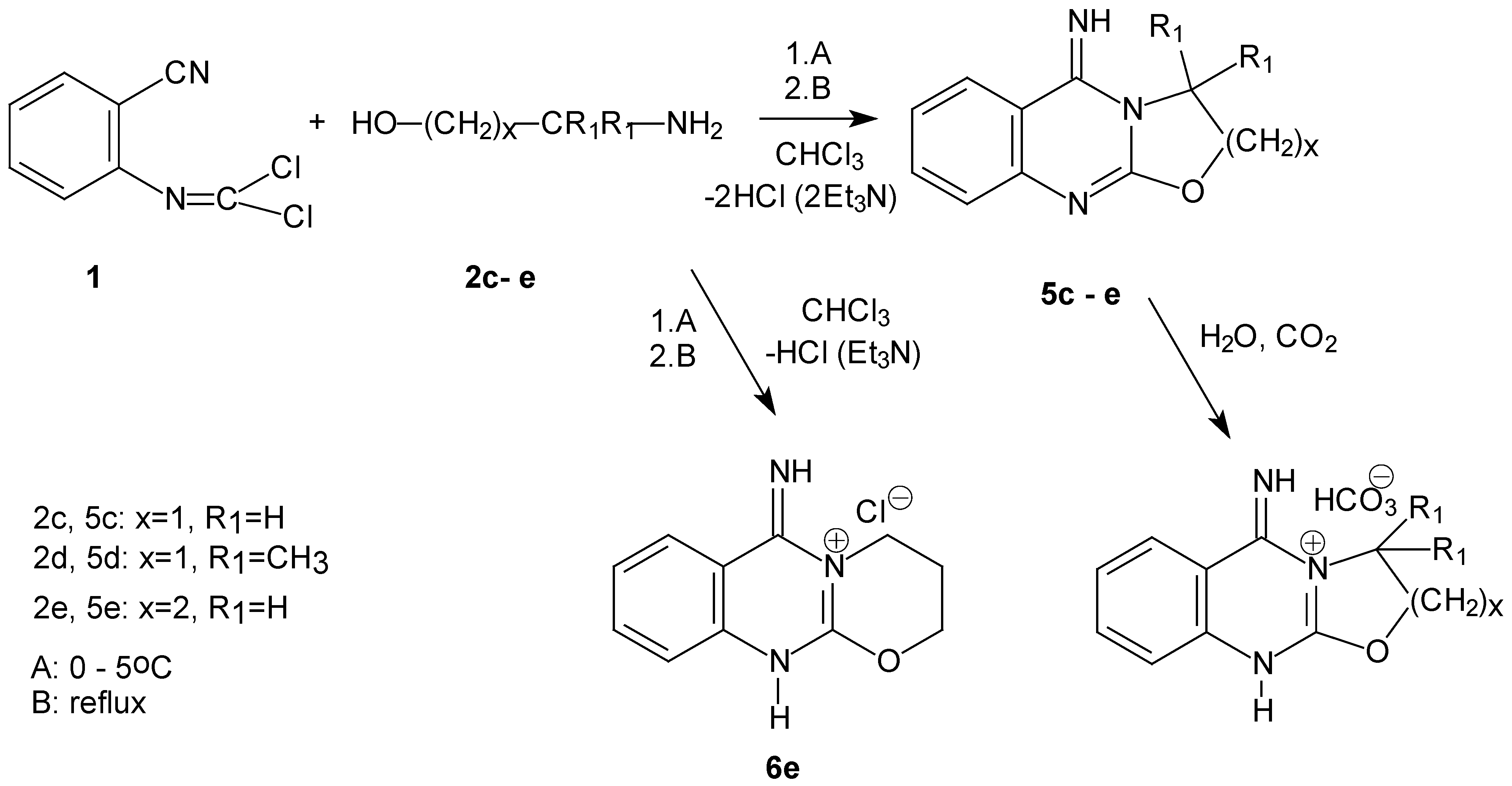
.Our attempt to detect intermediates of the reactions between
1 and 1,2- or 1,3-aminoalcohols
2c-2e described in
Scheme 3 was not successful. Using one equivalent of triethylamine, cooling, room temperature and heating, respectively, mixtures of
1, final fused quinazoline
5 and some other products were obtained. However, they undergo degradation in the course of their separation and highly polar products were formed. The trapping of the reaction intermediates was unsuccessful at lower temperature (–25 °C), regardless of the base used, amount, and duration of the reaction. 6-Imino-2
H, 3
H, 4
H, 6
H, 11
H-1,3-oxazino[2,3-b]-5-quinazolinium chloride (
6e) was isolated only in the case of the reaction of
1 with
2e.
We have found that these conditions are optimal for a two- step
one-pot synthesis: the first exothermic step was carried out with one equivalent of triethylamine at 0 – 5°C and the next step with the second equivalent of a base. The reaction stopped after the first step, unless
N-(2-cyanophenyl)- chloromethanimidoyl chloride (
1) was present in the reaction mixture. The reaction of amino alcohols can proceed
via the quinazoline intermediate formation or by formation of oxazolane/oxazinane imine intermediates, similar to reactions between amino alcohols and chloromethanimidoyl chlorides described in the literature [
12].
The identities of the products
5c – e and
6e were confirmed by elemental analysis, FTIR,
1H- and
13C NMR spectroscopy and in the case of
5d, by mass spectroscopy (
Scheme 4).
The corresponding vibration bands of the COC and C=N groups were found in the FTIR spectra of 5c – 5d. In the NMR spectra of 5c-5e and 6e the signals of aliphatic groups were observed. The salt 6e was identified as the 1,3-oxazino[2,3-b]-5-quinazolinium cation in accordance with the FTIR spectrum, in which N+-H valence vibrational bands and the isouronium moiety (absence of the band about 1650 cm-1) were not present. Furthermore, we have found that these imines 5c-5e form very stable hydrogen carbonates by action of atmospheric carbon dioxide and water. They were insoluble in water, dimethylsulfoxide, organic, inorganic acids and other solvents. But they afforded FTIR spectra with the same characteristics as in case of 6e. We expect that these hydrogen carbonates contained the corresponding 1,3-oxazolo[2,3-b]quinazoline-4-ium or 1,3-oxazino[2,3-b]-5-quinazolinium cation, respectively.
Experimental
General
Melting points of the compounds prepared were measured on a Boetius Rapido PHMK 79/2106 (Wägetechnik) instrument and are not corrected. TLC was carried out on Silufol UV 254 plates (Kavalier, Votice) and the detection with Fluotest Universal (Quarzlampen, Hanau) or by iodine vapors. Chloroform and diethyl ether were used as eluents. Purity of all compounds was determined by C, H, N elemental analysis on an Erba 1102 instrument. FTIR spectra were taken on a Genesis (UNICAM) spectrometer in potassium bromide pellets. NMR spectra were measured on a Bruker Avance DRX-500 spectrometer in deuterochloroform or deuterodimethyl sulfoxide. The 1H and 13C spectra were referenced to tetramethylsilane as the internal standard or to the solvent signal (deuterochloroform, hexadeuterodimethylsulfoxide). The measured 13C and 1H-NMR spectra were correlated with those obtained by on-line simulation (Advanced Chemistry Development, Inc., Toronto, Canada). Gas chromatography was accomplished on a Shimadzu GC – 17A apparatus and on a TRIO 1000 GC/MS system (FISONS Instruments). The electron impact method was used for ionization (70eV).
General procedure – Reactions of 1 with N,O-bifunctional nucleophiles 2
Part 1. A triethylamine (0.5 g, 5 mmol) solution in chloroform (10 mL) was added dropwise with stirring at 0 to - 5°C to mixture of N-(2-cyanophenyl)chloromethanimidoyl chloride (1) (1.0 g, 5 mmol) and the corresponding 2-aminophenol, 2-aminoethanol or 3-aminopropanol 2 (5 mmol) in chloroform (75 ml).
Part 2. The second equivalent of triethylamine was added after 3 hours of stirring. The solution was refluxed for 20 hours. The reaction mixture was cooled and washed with water and then dried with sodium sulfate. The filtrate was concentrated in vacuo. The residue was crystallized.
N-(2-cyanophenyl)-(2-hydroxyanilino)methanimidoyl chloride (3a)
2-Aminophenol (2a) (0.53 g, 5 mmol) and 1 equivalent of triethylamine were used in accord with the above described general procedure – Part 1. Yield 0.87 g (65 %); M.p. 142-144°C (benzene – cyclo-hexane); For C14H10ClN3O (271.21) calculated 61.97 % C, 3.72 % H, 15.49 % N; found 62.10 % C, 4.12 % H, 15.11 % N; FTIR, /cm-1: 3305 (NH), 2226 (CN), 1650 (C=N), 1237 (CO),770 (CCl); 1H-NMR (CDCl3) δ: 9.12 (0.5H, bs, NH), 8.64 (1H, bs, OH), 8.40 (0.5H, bs, NH), 7.82 – 7.64 (1H, m, ArH), 7.58 – 7.28 (4H, m, ArH), 7.09 – 6.93 (2H, m, ArH), 6.72- 6.54 (1H, m, ArH); 13C NMR (CDCl3) δ: 148.05 (Cq), 145.41 (Cq), 142.44 (Cq), 135.89 (CHAr), 134.22 (CHAr), 127.58 (Cq), 125.56 (CHAr), 123.44 (CHAr), 122.89 (CHAr), 122.13 (Cq), 119.68 (CHAr), 118.34 (CHAr), 117.94 (CHAr), 114.22 (Cq).
2-(3-Hydroxyphenyl)-2-chloro-3,4-dihydroquinazolin-4-imine (4a)
From 2-aminophenol (2a) (0.53 g, 5 mmol) and 2 equivalent of triethylamine. Yield 0,2 g (49%); M.p. 213-215° (acetone); For C14H10ClN3O (271.21) calculated 61.97 % C, 3.72 % H, 15.49 % N; found 61.68 % C, 3.34 % H, 15.70 % N; FTIR, /cm-1: 3450 (OH), 3300 (NH), 1660 (C=N), 1238 (CO), 655 (CCl); 1H-NMR (DMSO-d6) δ: 9.73 (1H, s, NH), 8.37 – 7.40 (8H, m, ArH), 4.22 (1H, s, OH); 13C NMR (DMSO) δ: 148.06 (Cq), 138.71 (Cq), 138.53 (Cq), 129.66 (Cq), 127.21 (CHAr), 122.03(Cq), 120.68 (CHAr), 119.57 (CHAr), 119.51 (CHAr), 118.86 (CHAr), 118.64 (CHAr), 111.83 (Cq), 110.35 (CHAr), 104.36 (CHAr); MS, m/z (Ir/%): 270 (39), 236 (39), 179 (15), 145 (2), 129 (29).
12H-Benzo[1,3]oxazolo[2,3-b]quinazolin-12-imine (5a)
To solution of 4a (1,0 g, 3 mmol) in dimethylformamide (1 mL) was added sodium hydride (0.2 g, 8 mmol). After 48 hours of stirring product 5a was precipitated by addition of water. Yield 0.7g (80 %); M.p. 236-237° C (dimethylformamide-water); For C14H9N3O (235.24) calculated: 71.48 % C, 3.86 % H, 17.85 % N; found: 71.85 % C, 3.76 % H, 17.54 % N; FTIR, /cm-1: 3210 (NH), 1620 (C=N), 1230, 1070 (COC); 1H-NMR (DMSO-d6) δ: 9.62 (1H, s, NH), 8.67 – 7.35 (8H, m, ArH), 13C NMR (DMSO) δ: 153.61(Cq), 153.45 (Cq), 144.26 (Cq), 144.07 (Cq), 132.76 (CHAr), 127.58 (Cq), 126.22 (CHAr), 125.11 (CHAr), 125.06 (CHAr), 124.40 (CHAr), 117.37 (Cq), 115.90 (CHAr), 109.91 (CHAr).
9-Chloro-12H-benzo[1,3]oxazolo[2,3-b]quinazolin-12-imine (5b)
From 4-chloro-2-aminophenol (2b) (0.7 g, 5 mmol) and 2 equivalents of triethylamine. Yield 0.9 g (69 %); M.p. 264-266°C (acetone); For C14H8ClN3O (269.69) calculated: 62.35 % C, 3.00 % H, 15.57 % N; found: 62,71 % C, 3.32 % H, 15.24 % N; FTIR, /cm-1: 3290 (NH), 1630 (C=N), 1245, 1085 (COC); 1H-NMR (DMSO-d6) δ: 9.57 (1H, s, NH), 8.51 – 7.40 (7H, m, ArH); 13C NMR (DMSO) δ: 153.50 (Cq), 152.43 (Cq), 143.86 (Cq), 143.27 (Cq), 135.11 (Cq), 132.93 (CHAr), 128.75 (Cq), 128.06 (CHAr), 126.35 (CHAr), 125.17 (CHAr), 124.66 (CHAr), 117.30 (CHAr), 115.51 (Cq), 111.20 (CHAr).
2,3-Dihydro-5H-[1,3]oxazolo]2,3-b]quinazolin-5-imine (5c)
From 2-aminoethanol (2c)(0.3 g, 5 mmol) and 2 equivalents of triethylamine. Yield 0.6 g (65 %); M.p. 210-211°C (dimethylformamide); For C10H9N3O (187.20) calculated: 64.16 % C, 4.86 % H, 22.44 % N; found: 64.55 % C, 4.61 % H, 22.78 % N; FTIR, /cm-1: 3300 (NH), 3030, 2980, 2890 (CH), 1620, 1650 (C=N), 1235, 1060 (COC); 1H-NMR (DMSO-d6) δ: 11.79 (1H, bs, NH), 7.47 – 7.38 (1H, m, ArH), 8.09 – 7.54 (3H, m, ArH), 4.49 – 4.15 (4H, m, CH2); 13C NMR (DMSO) δ: 160.20 (Cq), 156.84(Cq), 148.64 (Cq), 135.53 (CH), 126.76 (CH), 125.28 (CH), 124.60 (CHar), 115.82 (Cq), 66.34 (CH2), 48.03 (CH2).
2,3-Dihydro-5H-3,3-dimethyl-5H-[1,3]oxazolo[2,3-b]quinazolin-5-imine (5d)
From 2-amino-2-methylpropan-1-ol (2d) (0.45 g, 5 mmol) and 2 equivalents of triethylamine. Yield 0.85 g (70 %); M.p. 116-119°C (2-methoxyethanol); For C12H13N3O (215.25) calculated: 66.95 % C, 6.10 % H, 19.51 % N; found: 67.13 % C, 6,37 % H, 19.21 % N; FTIR, /cm-1: 3478, 3444 (NH), 3025, 2975, 2929 (CH), 1646 (C=N), 1216, 1054 (COC); 1H-NMR (DMSO-d6) δ: 8.76 – 7.53 (4H, m, ArH), 4.56 (2H, s, CH2), 1.84 (s, 6H, CH3); 13C NMR (DMSO) δ: 155.32 (Cq), 153.56 (Cq), 148.51 (Cq), 137.20 (CHar), 126.24 (CHar), 125.92 (CHar), 125.65 (CHar), 112.86 (Cq), 79.04 (CH2), 65.32 (Cq), 22.67 (CH3); MS, m/z (Ir/%): 215 (54), 200 (80), 186 (18), 157 (9), 143 (6), 129 (18).
3,4-Dihydro-2H,6H-[1,3]oxazino[2,3-b]quinazolin-6-imine (5e)
From 3-aminopropanol (2e) (0.41 g, 5 mmol) and 2 equivalents of triethylamine. Yield 0.60g (63 %); M.p. 128-130°C (ethanol)); For C11H11N3O (201.23) calculated: 65.65 % C, 5.52 % H, 20.87 % N; found: 66.0 % C, 5.71 % H, 20.47 % N; FTIR, /cm-1: 3369, 3245 (NH), 1656 (C=N), 1256, 1055 (COC); 1H-NMR (DMSO-d6) δ: 12.20 (0.3H, bs, NH), 11.52 (0.7H, bs, NH), 8.54 – 8.52 (1H, m, ArH), 7.82 – 7,79 (1H, m, ArH), 7.38 – 7.33 (1H, m, ArH), 3.99 – 3.97 (2H, m, OCH2), 3.60 – 3.58 (2H, m, NCH2), 2.12 – 2.10 (2H, m, CH2); 13C NMR (DMSO) δ: 175.54 (Cq), 159.74 (Cq), 139.50 (Cq), 135.84 (CHar), 127.72 (CHar), 124.90 (CHar), 116.02 (CHar), 115.95 (Cq), 59.40 (CH2O), 44.18 (CH2N), 30.38 (CH2).
6-Imino-2H, 3H, 4H, 6H, 11H-1,3-oxazino[2,3-b]quinazolin-5-ium chloride (6)
From 3-aminopropanol (2e) (0.41 g, 5 mmol) and 1 equivalent of triethylamine, as described in the general procedure – Part 1. Yield 0.71 g (60 %); M.p. 183-185°C (ethanol); For C11H12ClN3O (237.68) calculated: 55.58 % C, 5.10 % H, 17.67 % N; found: 55.95 % C, 5.42 % H, 17.50 % N; FTIR, /cm-1: 3240, 3280 (NH), 2960 (CH), 1620 (C=N), 1250, 1055 (COC); 1H-NMR (DMSO-d6) δ: 12.10 (1H, s, NH), 9.35 (1H, s, NH), 8.10 – 7.21 (4H, m, ArH), 4.62 – 4.60 (2H, m, OCH2), 3.46 – 3.42 (2H, m, N+CH2), 1.90 – 1.87 (2H, m, CH2); 13C NMR (DMSO) δ: 174.42 (Cq), 154.14 (C11a), 135.70 (Cq), 133.23 (CH), 126.07 (CHar), 124.05 (CHar), 115.66 (CHar), 114.62 (Cq), 58.76 (OCH2), 44.70 (N+CH2), 29.52 (CH2).