General details
Melting points of all the compounds were measured on a Boetius Rapido PHMK 79/2106 (Wägetechnik) instrument. TLC was carried out on Silufol UV 254 plates (Kavalier, Votice). The eluent used was a 20: 80 mixture of acetone: benzene. TLC detection was made by Fluotes Universal (Quarzlampen, Hanau) and iodine vapours, respectively. Purity of compounds 1-6 was proved by the elemental analysis on an Erba 1102 instrument. FTIR spectra ( /cm-1) were taken on a Genesis (Unicam) spectrometer in potassium bromide pellets. NMR spectra (δ/ppm) were measured on a Bruker Avance DRX-500 spectrometer. Unless stated otherwise, 1H- and 13C-NMR spectra were measured in CDCl3 and tetramethylsilane was used as an internal standard. The measured 13C and 1H NMR spectra were correlated with those obtained by on-line simulation (Advanced Chemistry Development, Inc., Toronto, Canada). Mass spectrometry was measured (electron impact, 70 eV) with FISONS Instruments TRIO 1000 and GC 8000 series.
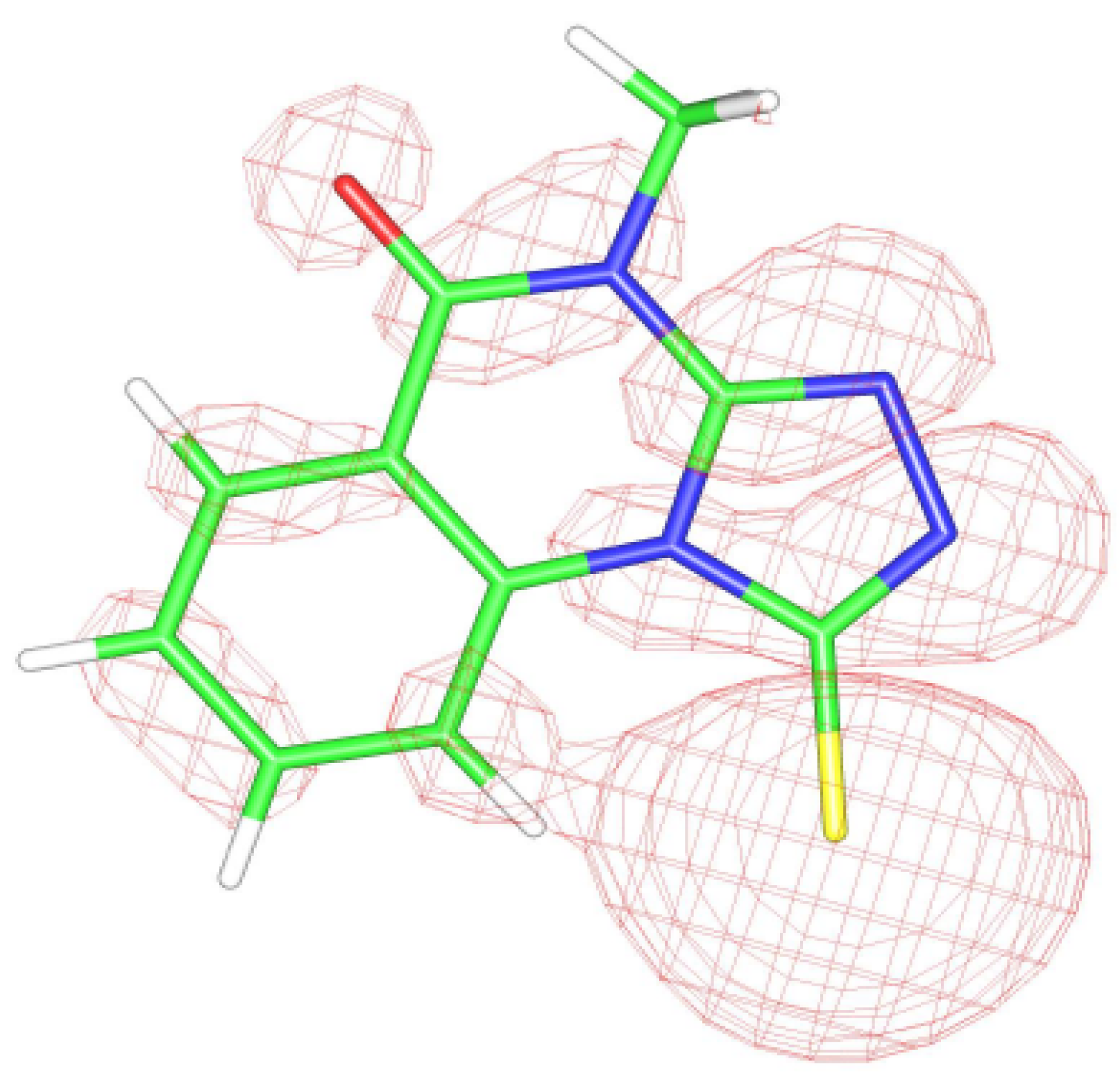
The theoretical results were obtained using the DFT method on the B3LYP/6-31-G* level [
5,
6]. Mulliken [
7], ESP [
8] and NBO [
9,
10,
11] methods were used to calculate the partial charges and properties of molecular orbitals. Mayer bond orders [
12] were calculated on a B3LYP/ii-iglo level using the Demon v.l program [
13]. The starting material
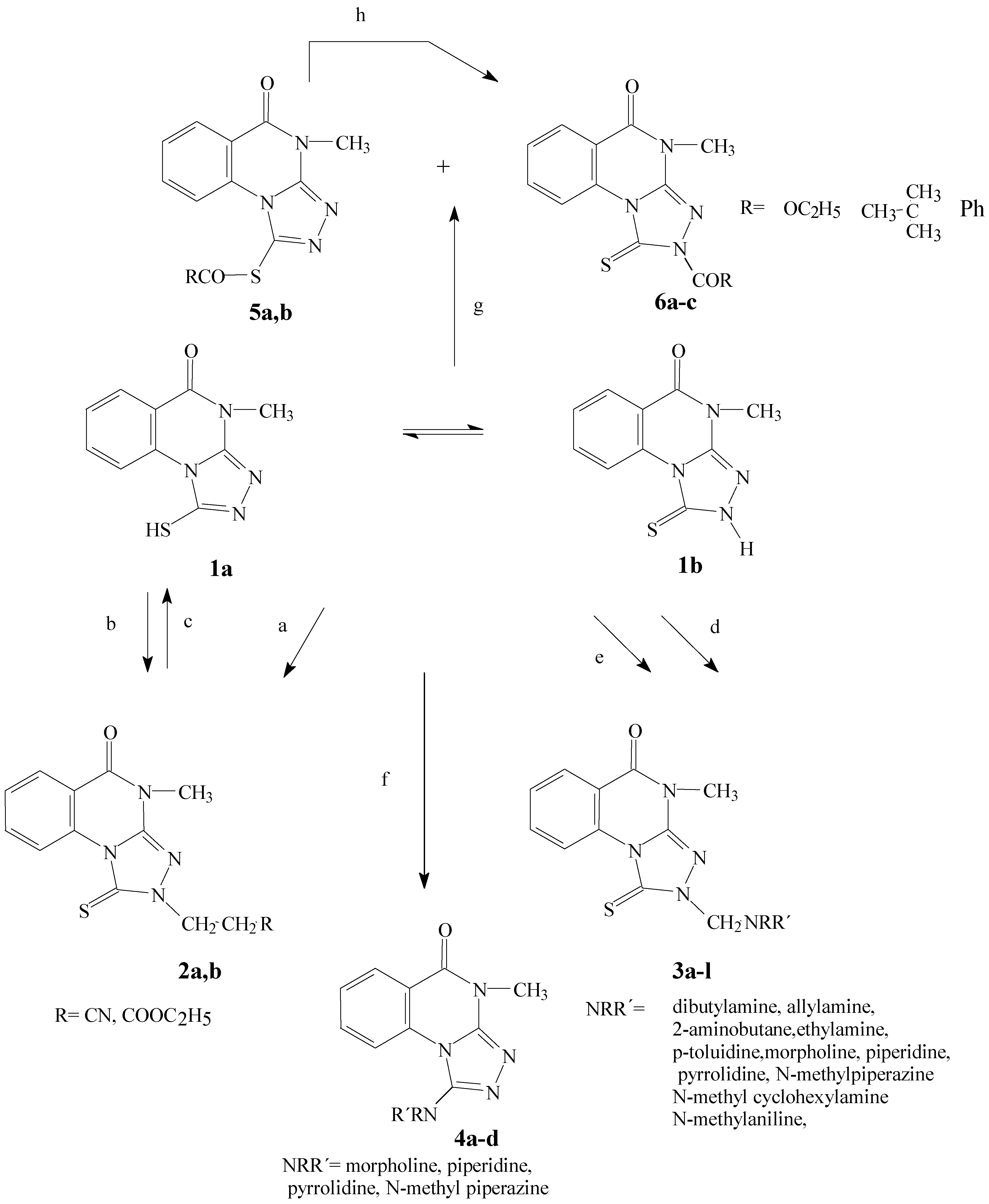
1 is prepared according to [
3] from 3-methyl-2-sulfanyl-3,4-dihydroquinazolin-4-one.
3-(4-Methyl-5-oxo-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-2-yl)propane derivatives 2a, 2b.
Michael reaction. Method A
To a mixture of triazolo derivative 1 (2.32 g, 0.01 mole) and triethyl amine (2 ml, 0.02 mole) in ethyl alcohol (30 mL, 95 %) the appropriate acrylic acid derivative (0.01 mole) was added. The reaction mixture was heated under reflux for 4-6h, concentrated under reduced pressure. The solid obtained was filtered, and crystallized from ethyl alcohol.
Method B
To a mixture of triazolo derivative 1 (2.32 g, 0.01 mole) and NaOH (O.8 g, 0.02 mole) in ethyl alcohol (30 mL, 95 %) the appropriate alkyl halide (namely ethyl chloropropionate and chloropropionitrile, 0.01 mole) was added. The reaction mixture was heated under reflux for 3-4 h, concentrated under reduced pressure. The solid obtained was filtered, and crystallized from ethyl alcohol.
Ethyl-3-(4-methyl-5-oxo-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-2-yl) propanoate (2a).
2.16 g (65%) (Method A, from 1), 1.43 g (45%) (Method B, from 1), M.p. 120-121 °C; For C15H16N4O3S (332.38) calculated: 54.21% C, 4.85% H, 16.86% N, 9.65% S, Found: 54.15% C, 4.76% H, 16.82% N, 9.57% S; FTIR: 1686(C=O), 1725 (C=O ester), 3087, 2977 (CH), 1631 (C=N); 1H-NMR: 10.33-7.51 (4H, m, ArH), 4.59 (1H, t, NCH2, J= 7.1 Hz), 4.18 (1H, q, OCH2, J= 7.1 Hz), 3.61 (3H, s, NCH3), 2.93 (2H, t, CH2CO, J= 7.1 Hz), 1.29 (3H, t, CH2CH3, J= 7.1 Hz); 13C-NMR: 170.51 (C=O), 161.72 (C=S), 158.36 (C=O, cyclic amide), 144.36 (Cq), 135.13 (Cq), 134.45 (CHAr), 129.00 (CHAr), 127.25 (CHAr), 116.92 (Cq), 116.55 (CHAr), 60.96 (NCH2), 44.32 (OCH2), 32.23 (CH2CO), 29.02 (NCH3), 14.17 (CH2CH3).
3-(4-Methyl-5-oxo-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-2-yl) propionitrile (2b).
2.13 g (75%) (Method A, from 1), 1.20g (40%) (Method B, from 1), M.p. 230-231 °C; For C13H11N5OS (285.32) calculated: 54.73% C, 3.89% H, 24.55% N, 11.24% S, Found: 54.69% C, 3.71% H, 24.45% N, 11.12% S; FTIR: 1687 (C=O), 2248 (CN), 3005, 2978, 2945 (CH), 1632 (C=N); 1H-NMR: 10.25-7.58 (4H, m, ArH), 4.59 (2H, t, NCH2, J= 6.85 Hz), 3.64 (3H, s, NCH3), 3.03 (2H, t, CH2CN, J= 6.85 Hz); 13C-NMR: 162.20 (C=S), 158.36 (C=O, cyclic amide), 144.76 (Cq), 135.13 (Cq), 134.56 (CHAr), 129.19 (CHAr), 127.55 (CHAr), 116.96 (Cq), 116.32 (CHAr), 115.23 (CN), 44.14 (NCH2), 29.10 (NCH3), 16.09 (CH2CN); Mass spectrum, m/z (Ir/%): 286 (18), 285 (100), 245 (34), 232 (29), 199 (3), 174 (5), 162 (12), 144 (8).
Mannich reaction
4-Methyl-2-(R,R´-aminomethyl)-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-ones (3a-l)
Method A
To the suspension of triazoloquinazoline 1 (2.32 g, 0.01 mole) in ethyl alcohol (30 mL, 95 %), formaldehyde (30%, 0.9 mL, 0.03mole) the desired amine (0.01 mole) were added dropwise with stirring. The reaction mixture was further stirred for 1-2h. at room temperature, concentrated under reduced pressure and cooled. The solid obtained was filtered and crystallized from ethyl alcohol.
Method B
To a mixture of triazolo derivative 1 (2.32 g, 0.01 mole) and triethyl amine (2 mL, 0.02 mole) in ethyl alcohol (30 mL, 95 %) the 4-(morpholinomethyl)morpholine (1.86 g, 0.01 mole) was added. The reaction mixture was heated under reflux for 3h, concentrated under reduced pressure. The solid obtained was filtered, and crystallized from ethyl alcohol.
4-Methyl-2-morpholinomethyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3a).
1.88 g (57%) (A, from 1), 1.49g (45%) (B, from 1), M.p. 185-186 °C; For C15H17N5O2S (331.39) calculated: 54.37% C, 5.17% H, 21.13% N, 9.67% S, Found: 54.23% C, 5.08% H, 20.98% N, 9.56% S; FTIR: 1688 (C=O), 2973, 2956 (CH), 1630 (C=N); 1H-NMR: 10.40-7.56 (4H, m, ArH), 5.22 (2H, s, NCH2N), 3.71 (4H, t, 2OCH2, J= 4.68 Hz), 3.64 (3H, s, NCH3), 2.89 (4H, t, 2NCH2, J= 4.68 Hz); 13C-NMR: 164.07 (C=S), 158.63 (C=O, cyclic amide), 144.53 (Cq), 135.44 (Cq), 134.59 (CHAr), 129.32 (CHAr), 127.52 (CHAr), 117.23 (Cq), 116.70 (CHAr), 69.26 (NCH2N), 67.12 (OCH2), 51.13 (NCH2), 29.31 (NCH3).
4-Methyl-2-piperidinomethyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3b).
1.68 g (51%), M.p. 210-211 °C; For C16H19N5OS (329.42) calculated: 58.30% C, 5.81% H, 21.26% N, 9.73% S, Found: 58.22% C, 5.88% H, 20.70% N, 9.65% S; FTIR: 1682 (C=O), 2932 (CH), 1629 (C=N); 1H-NMR: 10.42-7.52 (4H, m, ArH), 5.22 (2H, s, NCH2N), 3.64 (3H, s, NCH3), 2.82 (4H, t, 2NCH2, J= 5.35 Hz), 1.62-1.57 (4H, m, 2NCH2CH2), 1.42-1.37 (2H, m, 2NCH2CH2CH2) ; 13C-NMR: 163.82 (C=S), 158.68 (C=O, cyclic amide), 144.31 (Cq), 135.52 (Cq), 134.50 (CHAr), 129.22 (CHAr), 127.38 (CHAr), 117.22 (Cq), 116.71 (CHAr), 70.24 (NCH2N), 52.15 (NCH2), 29.29 (NCH3), 26.21 (NCH2CH2), 24.03 (NCH2CH2CH2).
4-Methyl-2-pyrrolidinomethyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3c).
1.92 g (61%), M.p. 196-197 °C; For C15H17N5OS (315.39) calculated: 57.12% C, 5.43% H, 22.21% N, 10.17% S, Found: 57.07% C, 5.21% H, 22.15% N, 9.95% S; FTIR: 1690 (C=O), 2970, 2950 (CH), 1630 (C=N); 1H-NMR: 10.42-7.52 (4H, m, ArH), 5.33 (2H, s, NCH2N), 3.64 (3H, s, NCH3), 2.95 (4H, t, 2NCH2, J= 6.31 Hz), 1.77 (4H, t, 2NCH2CH2); 13C-NMR: 163.73 (C=S), 158.67 (C=O, cyclic amide), 144.49 (Cq), 135.49 (Cq), 134.55 (CHAr), 129.22 (CHAr), 127.40 (CHAr), 117.21 (Cq), 116.73 (CHAr), 65.56 (NCH2N), 50.77 (NCH2), 29.29 (NCH3), 24.19(NCH2CH2).
4-Methyl-2-(4-methylpiperazino)methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3d).
1.54 g (45%), M.p. 184-185 °C; For C16H20N6OS (344..43) Calculated: 55.79% C, 5.85% H, 24.40% N, 9.31% S, Found: 55.68% C, 5.59% H, 24.34% N, 9.25% S; FTIR: 1693 (C=O), 2934 (CH), 1633 (C=N); 1H-NMR: 10.40-7.54 (4H, m, ArH), 5.24 (2H, s, NCH2N), 3.62 (3H, s, NCH3), 2.94 (4H, t, 2NCH2, J= 4.82 Hz), 2.46 (4H, t, 2NCH2CH2, J= 4.97 Hz), 2.27 (3H, s, NCH3); 13C-NMR: 164.01 (C=S), 158.64 (C=O, cyclic amide), 144.46 (Cq), 135.52 (Cq), 134.53 (CHAr), 129.33 (CHAr), 127.46 (CHAr), 117.27 (Cq), 116.76 (CHAr), 69.00 (NCH2N), 55.26 (NCH2), 50.58 (CH2NCH3), 46.23 (CH2NCH3), 29.24 (NCH3).
2-Cyclohexyl-N-methylaminomethyl-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3e).
1.78 g (50%), M.p. 141-142 °C; For C18H23N5OS (357.47) calculated: 60.48% C, 6.48% H, 19.59% N, 8.97% S, Found: 60.35% C, 6.45% H, 19.57% N, 8.82% S; FTIR: 1689 (C=O), 2947, 2928 (CH), 1628 (C=N); 1H-NMR: 10.43-7.54 (4H, m, ArH), 5.28 (2H, s, NCH2N), 3.64 (3H, s, NCH3), 2.72-2.89 (1H, m, NCH), 2.22 (3H, s, NCH3), 1.91-1.27 (10H, m, 5CH2); 13C- NMR: 163.04 (C=S), 158.74 (C=O, cyclic amide), 144.31 (Cq), 135.57 (Cq), 134.54 (CHAr), 129.22 (CHAr), 127.36 (CHAr), 117.25 (Cq), 116.84 (CHAr), 67.89 (NCH2N), 61.07 (NCH), 37.03 (CH2NCH3), 30.59 (NCHCH2), 29.28 (NCH3), 26.87 (CHCH2CH2), 26.01 (CHCH2CH2CH2).
4-Methyl-N-methylanilinomethyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3f).
1.40 g (40%), M.p. 178-179 °C; For C18H17N5OS (351.43) calculated: 61.52% C, 4.88% H, 19.93% N, 9.12% S, Found: 61.41% C, 4.73% H, 19.57% N, 8.92% S; FTIR: 1690 (C=O), 3045, 3006, 2939 (CH), 1631 (C=N); 1H-NMR: 10.36-6.82 (9H, m, ArH), 5.85 (2H, s, NCH2N), 357 (3H, s, NCH3), 3.32 (3H, s, NCH3); 13C-NMR: 162.68 (C=S), 158.60 (C=O, cyclic amide), 147.38 (Cq), 144.74 (Cq), 135.40 (Cq), 134.59 (CHAr), 129.42 (CHAr), 129.29 (CHAr), 127.45 (CHAr), 118.95 (CHAr), 117.23 (Cq), 116.77 (CHAr), 113.87 (CHAr), 65.70 (NCH2N), 39.67 (CH2NCH3), 29.25 (NCH3).
2-(Dibutylamino)methyl-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3g).
2.23 g (60%), M.p. 118-119 °C; For C19H27N5OS (373.52) calculated: 61.10% C, 7.29% H, 18.75% N, 8.58% S, Found: 59.89% C, 7.18% H, 18.65% N, 8.31% S; FTIR: 1690 (C=O), 2955, 2930, 2870 (CH), 1629 (C=N); 1H-NMR: 10.45-7.52 (4H, m, ArH), 5.28 (2H, s, NCH2N), 3.63 (3H, s, NCH3), 2.77 (4H, t, 2NCH2, J= 7.27 Hz), 1.48-1.59 (4H, m, 2NCH2CH2), 1.21-1.39 (4H, m, 2NCH2CH2CH2), 0.94 (3H, t, 2CH2CH3, J= 7.26 Hz); ; 13C-NMR: 163.47 (C=S), 158.75 (C=O, cyclic amide), 144.39 (Cq), 135.58 (Cq), 134.50 (CHAr), 129.24 (CHAr), 127.35 (CHAr), 117.25 (Cq), 116.81 (CHAr), 66.63 (NCH2N), 52.29 (NCH2), 30.40 (NCH2CH2), 29.24 (NCH3), 20.63 (NCH2CH2CH2), 14.24 (CH2CH3).
2-(Ethylamino)methyl-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3h).
1.59 g (55%), M.p. 165-166 °C; For C13H15N5OS (289.35) calculated: 53.96% C, 5.22% H, 24.20% N, 11.08% S, Found: 53.86% C, 4.97% H, 23.87% N, 10.93% S; FTIR: 1691 (C=O), 2969, 2956 (CH), 1628 (C=N), 3390 (NH) ; 1H-NMR: 10.38-7.53 (4H, m, ArH), 5.20 (2H, d, NCH2NH), 3.64 (3H, s, NCH3), 2.68 (2H, q, NCH2, J= 7.13 Hz), 1.11 (3H, t, CH2CH3, J= 7.13 Hz); ; 13C-NMR: 162.81 (C=S), 158.64 (C=O, cyclic amide), 144.51 (Cq), 135.46 (Cq), 134.62 (CHAr), 129.29 (CHAr), 127.46 (CHAr), 117.20 (Cq), 116.77 (CHAr), 64.16 (NCH2N), 40.58 (NCH2), 29.28 (NCH3), 15.09 (NCH2CH3).
2-(Allylamino)methyl-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3i).
1.05 g (35%), M.p. 139-140 °C; For C14H15N5OS (301.37) calculated: 55.80% C, 5.22% H, 23.24% N, 10.64% S, Found: 55.61% C, 4.97% H, 23.12% N, 10.52% S; FTIR: 1691 (C=O), 2973, 2956 (CH), 1629 (C=N), 3340 (NH) ; 1H-NMR: 10.34-7.54 (4H, m, ArH), 5.93-5.71 (1H, m, CH=CH2), 5.24 (1H, d, CH=CH2, J= 10.53 Hz), 5.20 (2H, d, NCH2NH, J= 2.93 Hz), 5.09 (1H, d, CH=CH2, J= 10.53 Hz), 3.63 (3H, s, NCH3), 3.31-3.28 (2H, d, HNCH2CH, J= 5.84 Hz); ; 13C- NMR: 163.82 (C=S), 158.41 (C=O, cyclic amide), 144.31 (Cq), 135.23 (Cq), 135.58 (CH=CH2) 134.41 (CHAr), 129.31 (CHAr), 127.42 (CHAr), 117.24 (Cq), 116.56 (CH=CH2), 116.52 (CHAr), 63.62 (NCH2N), 48.64 (NCH2), 29.26 (NCH3).
2-(sec-Butylamino)methyl-4-methyl--1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3j).
1.77 g (56%), M.p. 133-134 °C; For C15H19N5OS (317.41) calculated: 56.76% C, 6.03% H, 22.06% N, 10.10% S, Found: 56.54% C, 5.83% H, 2186% N, 9.77% S; FTIR: 1690 (C=O), 2965, 2926 (CH), 1627 (C=N), 3365 (NH) ; 1H-NMR: 10.38-7.54 (4H, m, ArH), 5.22 (2H, d, NCH2NH, J= 2.93 Hz), 3.64 (3H, s, NCH3), 2.52-2.63 (1H, m, NCH), 1.58-1.32 (2H, m, NCHCH2), 1.09 (3H, d, CHCH3, J= 6.43 Hz), 0.89 (3H, t, CH2CH3, J= 7.47 Hz) ; 13C-NMR: 162.06 (C=S), 158.67 (C=O, cyclic amide), 144.50 (Cq), 135.50 (Cq), 134.63 (CHAr), 129.27 (CHAr), 127.44 (CHAr), 117.22 (Cq), 116.80 (CHAr), 62.10 (NCH2N), 50.37 (NCH), 52.29 (NCH2), 29.59 (NCHCH2), 29.23 (NCH3), 19.58 (CHCH3), 10.20 (CH2CH3).
4-Methyl-1-thioxo-2-(p-toluidinomethyl)-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3k).
1.77 g (65%), M.p. 171-172 °C; For C18H17N5OS (351.43) calculated: 61.52% C, 4.88% H, 19.93% N, 9.12% S, Found: 61.42% C, 4.76% H, 19.82% N, 9.01% S; FTIR: 1691 (C=O), 2952, 2928 (CH), 1628 (C=N), 3385 (NH) ; 1H-NMR: 10.28-6.84 (8H, m, ArH), 5.64 (2H, d, NCH2NH), 3.59 (3H, s, NCH3), 2.21 (3H, s, NCH3); 13C-NMR (CDCl3): 162.19 (C=S), 158.53 (C=O, cyclic amide), 142.27 (Cq), 135.30 (Cq), 134.57 (CHAr), 130.02 (CHAr), 129.26 (CHAr), 129.13 (Cq), 127.48 (CHAr), 117.12 (Cq), 116.69 (CHAr), 114.69 (CHAr), 58.79 (NCH2N), 29.23 (NCH3), 20.60 (PhCH3).
2-(Diethylamino)methyl-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (3l).
1.39 g (45%), M.p. 160-161°C; For C15H19N5OS (317.41) calculated: 56.76% C, 6.03% H, 22.06% N, 10.10% S, Found: 56.59% C, 5.87% H, 21.91% N, 9.92% S; FTIR: 1689 (C=O), 2985, 2960 (CH), 1630 (C=N); 1H-NMR: 10.37-7.42 (4H, m, ArH), 5.22 (2H, s, NCH2N), 3.56 (3H, s, NCH3), 2.79 (4H, q, 2NCH2, J= 7.31 Hz), 1.11 (6H, t, 2NCH2CH3, J= 7.0 Hz); 13C-NMR: 163.46 (C=S), 158.70 (C=O, cyclic amide), 144.46 (Cq), 135.53 (Cq), 134.50 (CHAr), 129.22 (CHAr), 127.35 (CHAr), 117.24 (Cq), 116.77 (CHAr), 65.63 (NCH2N), 46.17 (NCH2), 29.25 (NCH3), 13.44 (NCH2CH3).
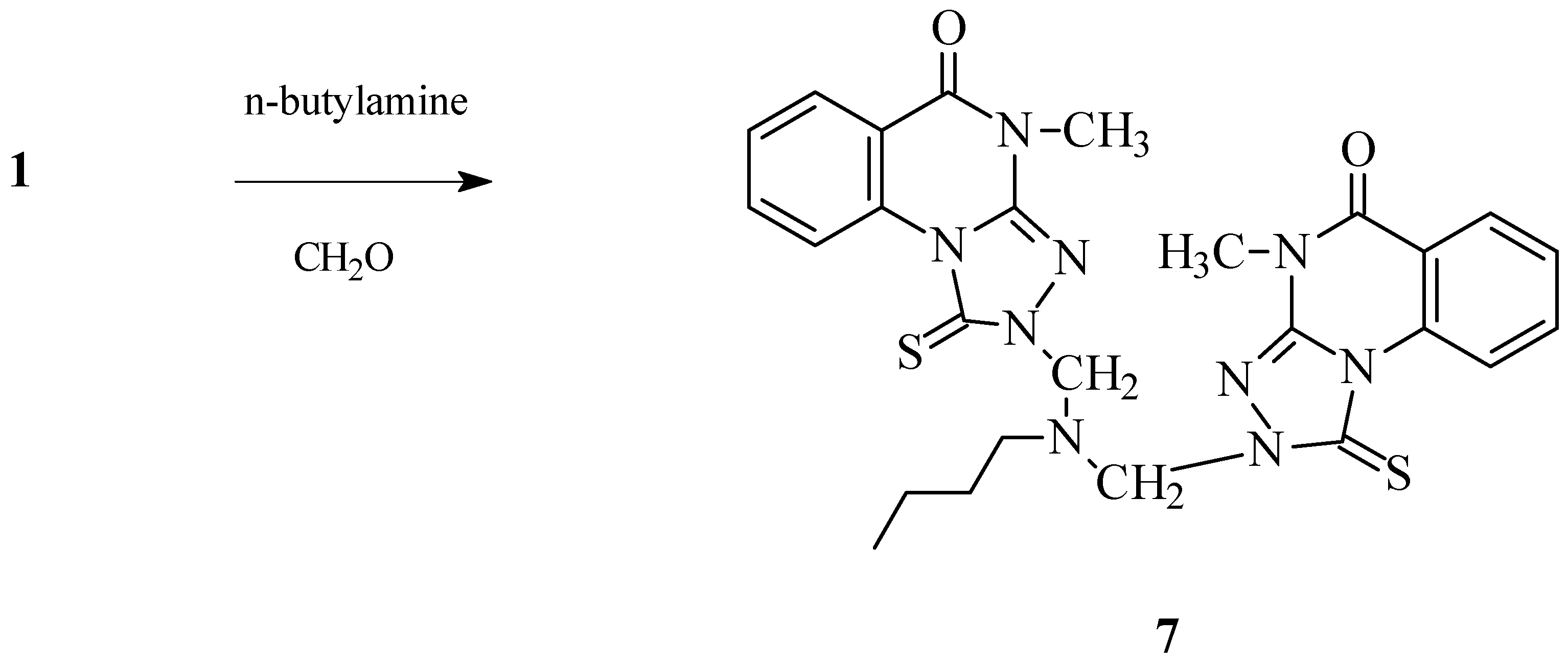
2-[Butyl[(4-methyl-5-oxo-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-2-yl)methyl]amino]methyl-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (7).
1.79 g (32%), M.p. 163-164 °C; For C26H27N9O2S2 (561.68) calculated: 55.60% C, 4.85% H, 22.44% N, 11.42% S, Found: 55.43% C, 4.63% H, 22.39% N, 11.31% S; FTIR: 1691 (C=O), 2952, 2928 (CH), 1627 (C=N); 1H-NMR: 10.36-7.53 (8H, m, ArH), 5.61 (4H, s, NCH2N), 3.60 (6H, s, NCH3), 3.04 (2H, t, NCH2, J= 7.26 Hz), 1.66-1.57 (2H, m, NCH2CH2), 1.35-1.21 (2H, m, NCH2CH2CH2), 0.87 (3H, t, CH2CH3, J= 7.25 Hz); 13C-NMR: 163 162.73 (C=S), 158.55 (C=O, cyclic amide), 144.56 (Cq), 135.37 (Cq), 134.62 (CHAr), 129.26 (CHAr), 127.48 (CHAr), 117.11 (Cq), 116.6 (CHAr), 67.00 (NCH2N), 50.87 (NCH2), 30.27 (NCH2CH2), 29.25 (NCH3), 20.20 (NCH2CH2CH2), 14.06 (CH2CH3).
Aminolysis reaction: General procedure
To the solution of triazoloquinazoline 1 (2.32 g, 0.01 mole) in the appropriate amine (10 mL), 5 drops of hydrogen peroxide were added drop wise during stirring. The reaction mixture was further stirred for 2-24h. At room temperature, to give a white precipitate, filter, wash with H2O and crystallized from ethyl alcohol.
4-Methyl-1-morpholino-4,5-dihydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (4a).
1.60 g (56%), M.p. 166-167°C; For C14H15N5O2 (285.30) calculated: 58.94% C, 5.30% H, 24.55% N, Found: 58.67% C, 5.21% H, 24.48% N; FTIR: 1686 (C=O), 2983, 2948 (CH), 1611 (C=N); 1H-NMR: 8.65-7.54 (4H, m, ArH), 4.12 (4H, t, 2OCH2, J= 4.87 Hz), 3.82 (3H, s, NCH3), 2.89 (4H, t, 2NCH2, J= 4.97 Hz); 13C-NMR: 158.77 (C=O, cyclic amide), 149.81 (Cq), 144.58 (Cq), 134.59 (CHAr), 133.96 (Cq), 130.01 (CHAr), 127.02 (CHAr), 117.54 (Cq), 116.96 (CHAr), 67.61 (OCH2), 51.72 (NCH2), 29.83 (NCH3).
4-Methyl-1-piperidino-4,5-dihydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (4b).
1.33 g (47%), M.p. 158-159°C; For C15H17N5O (283.33) calculated: 63.59% C, 6.05% H, 24.72% N, Found: 63.45% C, 5.98% H, 24.65% N; FTIR: 1689 (C=O), 2985, 2932 (CH), 1613 (C=N); 1H-NMR: 8.65-7.54 (4H, m, ArH), 3.82 (3H, s, NCH3), 3.17 (4H, t, 2NCH2, J= 5.40 Hz), 1.54-1.68 (4H, m, 2NCH2CH2), 1.41-1.29 (2H, m, NCH2CH2CH2); 13C-NMR: 158.77 (C=O, cyclic amide), 149.81 (Cq), 144.58 (Cq), 134.59 (CHAr), 133.96 (Cq), 130.01 (CHAr), 127.02 (CHAr), 117.54 (Cq), 116.96 (CHAr), 57.62 (NCH2), 29.64 (NCH3), 27.28 (NCH2CH2), 22.58 (NCH2CH2CH2).
4-Methyl-1-pyrrolidino-4,5-dihydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (4c).
1.31 g (49%), M.p. 163-164°C; For C14H15N5O (269.305) calculated: 62.44% C, 5.61% H, 26.01% N, Found: 62.23% C, 5.56% H, 26.01% N; FTIR: 1684 (C=O), 2987 (CH), 1611 (C=N); 1H-NMR: 8.72-7.58 (4H, m, ArH), 3.62 (3H, s, NCH3), 3.06 (4H, m, 2NCH2), 1.75-1.68 (4H, m, 2NCH2CH2); 13C-NMR: 158.37 (C=O, cyclic amide), 149.75 (Cq), 142.89 (Cq), 134.66 (CHAr), 133.67 (Cq), 128.87 (CHAr), 126.85 (CHAr), 117.31(Cq), 116.59 (CHAr), 54.45 (NCH2), 29.13 (NCH3), 24.36 (NCH2CH2).
4-Methyl-1-(4-methylpiperazino)-4,5-dihydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (4d).
1.33 g (53%), M.p. 167-168°C; For C15H18N6O (298.35) calculated: 60.39% C, 6.08% H, 28.17% N, Found: 60.21% C, 5.85% H, 27.76% N; FTIR: 1689 (C=O), 2985 (CH), 1613 (C=N); 1H-NMR: 8.91-7.54 (4H, m, ArH), 3.81 (3H, s, NCH3), 3.11 (4H, t, 2NCH2, J= 4.68 Hz), 2.14 (4H, t, 2NCH2CH2, J= 4.68 Hz), 2.20 (3H, s, CH2NCH3); 13C NMR: 159.05 (C=O, cyclic amide), 150.39 (Cq), 143.17 (Cq), 134.87 (CHAr), 134.40 (Cq), 130.47 (CHAr), 127.56 (CHAr), 118.00 (Cq), 117.17 (CHAr), 56.29 (NCH2), 56.10 (NCH2CH2N), 46.13 (CH2NCH3), 29.99 (NCH3)
Acylation reaction. General procedure
The appropriate acyl chloride (ethyl chloroformate for 5a, 6a, pivaloyl chloride for 5b, 6b or benzoyl chloride for 6c) (0.01 mole) was added to a mixture of triazolo derivative 1 (2.32 g, 0.01 mole) and triethyl amine (2 mL, 0.02 mole) solution in benzene (30 mL). The reaction mixture was heated under reflux for 15 min. Concentrated under reduced pressure. The solid obtained was filtered, and crystallized from ethyl alcohol. The obtained product was a mixture of both 5 and 6 and was not further purified. The constitutional isomers purity was determined from the 1H-NMR spectra. Compounds 5a and 5b were not isolated but detected only from the 1-NMR spectra, IR spectroscopy and by the comparison with the 1H-NMR spectra of the pure 6a and 6b. The chloroform solution of the mixture of 5 and 6 were heated under reflux for 1h. to give the pure N-acyl derivatives 6.
Ethyl-[(4-methyl-5-oxo-4,5-dihydro[1,2,4]triazolo[4,3-a]quinazolin-1-yl)sulfanyl]methanoate (5a).
FTIR: 1708 (C=O), 1743 (C=O ester), 3063, 2980 (CH), 1635 (C=N); 1H-NMR: 8,72-7.34 (4H, m, ArH), 4.33 (2H, q, OCH2, J= 7.0 Hz), 3,82 (3H, s, NCH3), 1,29 (3H, t, CH2CH3, J= 7.0 Hz).
4-Methyl-5-oxo-4,5-dihydro[1,2,4]triazolo[4,3-a]quinazolin-1-yl-2,2dimethylpropanethioate (5b).
FTIR: 1691(C=O), 1743 (C=O), 3002, 2978 (CH), 1645 (C=N); 1H-NMR: 8,62-7.56 (4H, m, ArH), 3,84 (3H, s, NCH3), 1,61 (9H, s, C(CH3)3).
Ethyl-4-methyl-5-oxo-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-2-carboxylate (6a).
1.64 g (54%), M.p. °C; For C13H12N4O3S (304.32) calculated: 51.31% C, 3.97% H, 18.41% N, 10.53% S, Found: 51.21% C, 3.95% H, 18.33% N, 10.42% S; FTIR: 1689 (C=O), 1778 (C=O ester), 3063, 2980 (CH), 1635 (C=N); 1H-NMR: 10.38-7.55 (4H, m, ArH), 4.55 (2H, q, OCH2, J= 7.0 Hz), 3.66 (3H, s, NCH3), 1.50 (3H, t, CH2CH3, J= 7.0 Hz); 13C-NMR: 176.08 (C=O), 164.75 (C=S), 158.48 (C=O, cyclic amide), 149.04 (Cq), 145.01 (Cq), 135.18 (Cq), 134.85 (CHAr), 129.47 (CHAr), 127.91 (CHAr), 117.17 (Cq), 116.55 (CHAr), 65.30 (OCH2), 29.36 (NCH3), 14.14.36 (CH2CH3).
2-(2,2-Dimethylpropanoyl)-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (6b).
1.42 g (45%), M.p. °C; For C15H16N4O2S (316.38) calculated: 56.95% C, 5.10% H, 17.71% N, 10.13% S, Found: 56.73% C, 4.97% H, 17.65% N, 9.96% S; FTIR: 1691(C=O), 1743 (C=O), 3002, 2978 (CH), 1645 (C=N); 1H-NMR: 10.49-7.56 (4H, m, ArH), 3.67 (3H, s, NCH3), 1.52 (9H, s, C(CH3)3); 13C-NMR: 176.08 (C=O), 164.71 (C=S), 158.65 (C=O, cyclic amide), 143.89 (Cq), 135.20 (Cq), 134.79 (CHAr), 129.50 (CHAr), 127.88 (CHAr), 117.09 (Cq), 116.84 (CHAr), 29.87 (C(CH3)3), 29.24 (NCH3), 27.40 (C(CH3)3).
2-Benzoyl-4-methyl-1-thioxo-1,2,4,5-tetrahydro[1,2,4]triazolo[4,3-a]quinazolin-5-one (6c).
1.17 g (35%), M.p. °C; For C17H12N4O2S (336.37) calculated: 60.70% C, 3.60% H, 16.66% N, 9.53% S, Found: 60.59% C, 3.45% H, 16.57% N, 9.49% S; FTIR: 1693 (C=O), 1720 (C=O), 2987, 2955 (CH), 1640 (C=N); 1H-NMR: 10.37-7.48 (9H, m, ArH), 3.60 (3H, s, NCH3); 13C-NMR: 166.40 (C=O), 165.04 (C=S), 158.55 (C=O, cyclic amide), 142.41 (Cq), 135.08 (Cq), 134.76 (CHAr), 134.01 (CHAr), 131.98 (CHq), 131.14 (CHAr), 129.46 (CHAr), 128.57 (CHAr), 127.92 (CHAr), 117.09 (Cq), 116.66 (CHAr), 29.32 (NCH3).