The Relationship between Redox Potentials and Torsional Angles in 4,4’-Dimethyl N, N’-Alkylidene 2,2’-Bipyridinium Salts
Abstract
:Introduction
- 1).
- The redox center is substituted with substituents of different electronegativity;
- 2).
- The conjugated system in the redox center is extended by substitution or another method, and
- 3).
- By changing the conformation of the redox molecule.
Results and Discussion
Experimental
Molecular structure building, energy minimization and geometry parameters measurement with AccuModel 1.2
- 1).
- Building of a molecular model using Accumodel 1.2 .
- 2).
- An energy minimization operation to obtain a molecular structure in the lowest energy level was performed.
- 3).
- Torsional angles between two pyridine rings and the total energy was calculated.
- 4).
- Other geometry and electronic parameters of the molecular models was calculated.
Synthesis of 4, 4’ –dimethyl, N, N’-ethylene-2, 2’-bipyridine (1) and 4, 4’ –dimethyl, N, N’-propylene 2, 2’-bipyridine(2)
Cyclic Voltammograms:
Acknowledgements
References
- Marfurt, J.; Wenyuan, Z.; Walder, L. J. Chem. Soc. Chem. Commun. 1994, 51–71. [CrossRef]
- Wenyuan, Z.; Xiangying, M.; Yijun, W.; Walder, L. J. Qingdao Uni. (Science) 1996, 9, 51–63.
- Wenyuan, Z.; Yijun, W.; Walder, L. J. Qingdao Uni. (Engineering) 1996, 11, 1–12.
- Wenyuan, Z. Synthesis of electropolymerizable bipyridinium salts and aromatic diimides used as electrode modifers, Bern University, 1992.
- Wenyuan, Z.; Marfurt, J.; Walder, L. Helv. Chim. Acta. 1994, 77(1), 351–71.
- Wenyuan, Z.; Yijun, W. Symposium of the 2th East Asian Polymer Conference, Hong Kong, 1998; p. 263.
- Wenyuan, Z.; Yijun, W.; Meng, X.; Walder, L. J. Qingdao Uni. (Engineering) 1996, 11, 1–12.
- Sample Availability: Samples are available from the authors.
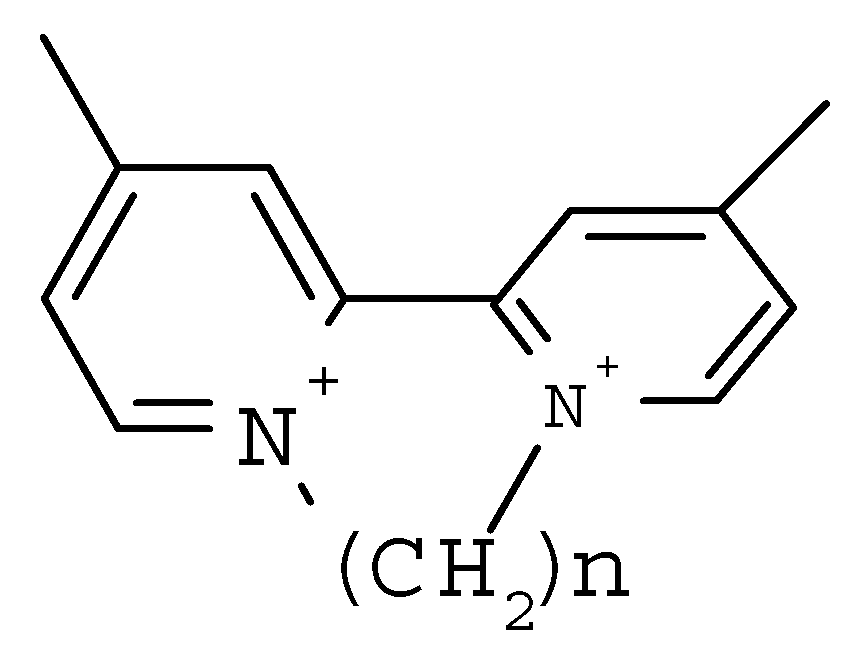
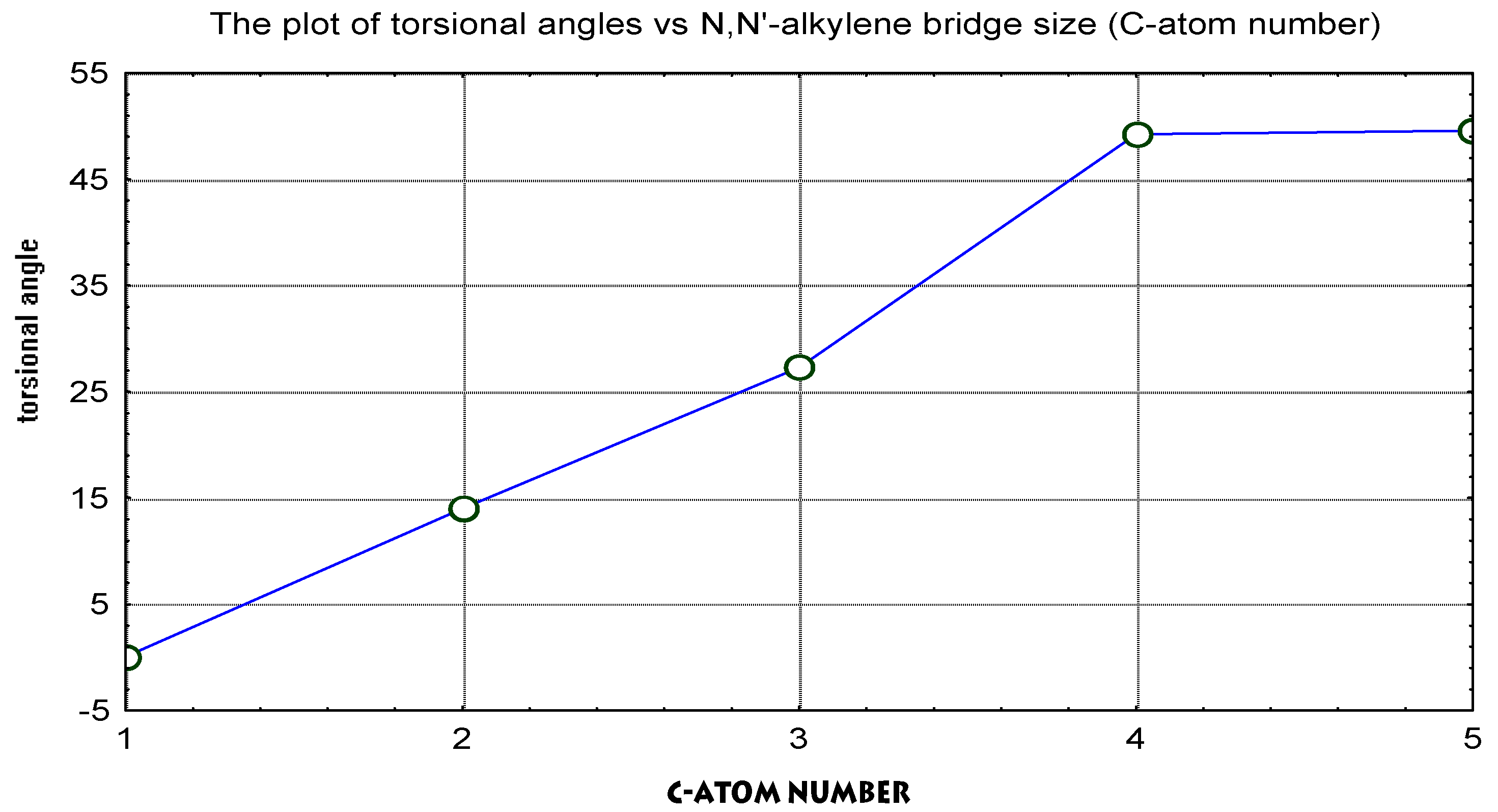
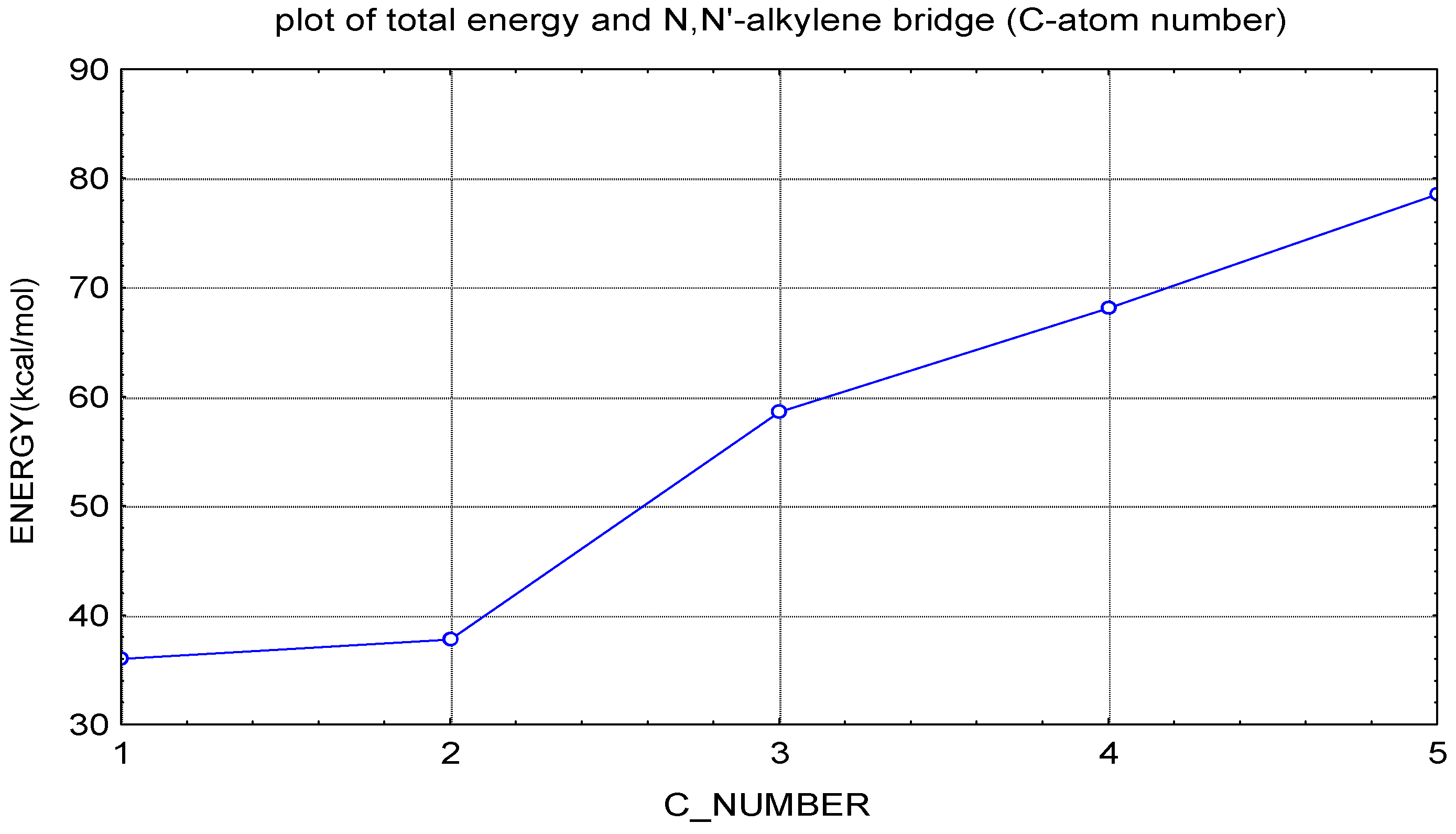
| No | Molecule Structure | Torsional angle (degrees) | Redox potential (measured) (volts vs. SCE) | Dipole moment | Total energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 |  | 0.02 | 0.0797 | 36 | |
| 2 | 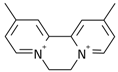 | 14.07 | 0.44 | 0.0219 | 37.8 |
| 3 |  | 27.30 | 0.66 | 0.0435 | 58.6 |
| 4 | 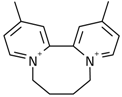 | 49.27 | 0.0677 | 68.1 | |
| 5 | 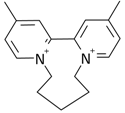 | 49.60 | 0.0208 | 78.5 |
| Minimization method | Steepest Descent method |
|---|---|
| Maximum number of calculation | 1000 |
| Number of Steps Between Screen Change | 5 |
| Convergency criterion (RMS force) | 0.05 |
| Initial Step | 0.0005 |
| Applied force field | MM3 + UFF(Universal force field) |
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Wang, Y.; Zhao, W. The Relationship between Redox Potentials and Torsional Angles in 4,4’-Dimethyl N, N’-Alkylidene 2,2’-Bipyridinium Salts. Molecules 2000, 5, 1379-1385. https://doi.org/10.3390/51201379
Wang Y, Zhao W. The Relationship between Redox Potentials and Torsional Angles in 4,4’-Dimethyl N, N’-Alkylidene 2,2’-Bipyridinium Salts. Molecules. 2000; 5(12):1379-1385. https://doi.org/10.3390/51201379
Chicago/Turabian StyleWang, Yijun, and Wenyuan Zhao. 2000. "The Relationship between Redox Potentials and Torsional Angles in 4,4’-Dimethyl N, N’-Alkylidene 2,2’-Bipyridinium Salts" Molecules 5, no. 12: 1379-1385. https://doi.org/10.3390/51201379




