General
Solvents and reagents were purified and dried according to standard procedures. TLC analyses were carried out with Si60F254-coated aluminium sheets (E.Merck) using ethylacetate/ i-hexane mixtures; detection by UV at 254 nm, phosphomolybdic acid (10% in ethanol) or sulfuric acid (40% in water). Silica gel 32-63 μm (Woelm) was used for flash chromatography, eluents as above. Melting points were determined on a Kofler hot block and are uncorrected. The optical rotations were measured on a POLAR L-μP (IBZ Messtechnik) polarimeter at 589 nm. IR spectra were recorded on a PU 9800 FTIR spectrometer (Philips Analytical) in film or KBr discs (0.5 mg of sample and 300 mg of KBr). 1H- NMR and 13C NMR spectra were obtained on a Varian model VXR 300 spectrometer (at 300.3 MHz and 75.12 MHz, respectively). In the NMR experiments deuterochloroform solutions with tetramethylsilane as internal standard were measured; evaluation of 1H-NMR spectra was according to 1st order interpretation; and multiplicity of 13C-NMR signals was deduced from broad-decoupled or DEPT spectra. The ratios of diastereomers 7 and 8 were established by comparison of the integrals of H-1 signals in 1H NMR experiments performed on crude reaction mixtures.
3-O-[(tButyl)-dimethylsilyl]-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose 4c
To a stirred solution of bisacetonide glucose (4a, 4g, 15 mmol) and triethylamine (2.4 mL,17 mmol, 1.1 equiv) in dry dimethylformamide (20 mL), kept at 0-5 °C, a solution of (tbutyl)- dimethylsilylchloride (2.562 g, 17 mmol, 1.1 equiv) in dry dimethylformamide (30 mL) was added dropwise over 30 min. The mixture was stirred at room temperature for another 30 h, then poured on ice/ water (100 mL) and extracted with chloroform (3 x 50 mL). The organic solutes were combined, dried (Na2SO4) and concentrated (rotary evaporator). The residue was treated with isohexane and unsoluble starting 4a (1 g) filtered off. The crude product after solvent removal was purified by flash chromatography (silica gel 80 g, eluent ethylacetate - isohexane 1:1). Yield 4.15 g (74%), Rf=0.68 (AcOEt - i-hexane 1:1), =-17.2 (c=0.43, CHCl3).
Spectral Data
1H-NMR (CDCl3) δ: 5.80 (d, 1H, H-1, J1,2=3.5 Hz), 4.28 (d, 1H, H-2, J1,2=3.5 Hz), 4.16 (m, 2H, H-3, H-5), 4.04 (dd, 1H, H-4, J3,4=6.1 Hz, J4,5=8.4 Hz), 3.96 (dd, 1H, H-6, B of ABX, JA,B=8.3 Hz, JB,X=2.7 Hz), 3.88 (dd, 1H, H-6, A of ABX, JA,B=8.3 Hz, JA,X=5.9 Hz), 0.85 (s, 9H, C(CH3)3), 0.07, 0.08 (all s, 3H, Si(CH3)2).
13C-NMR ( CDCl3) δ: 111.7 (s, C(CH3)2), 108.8 (s, C(CH3)2), 105.2 (d, C-1), 85.6, 82.2, 75.4, 72.1 (all d, C-2, C-3, C-4, C-5), 67.7 (t, C-6), 29.9, 26.7, 26.3, 25.2 (all q, C(CH3)2), 25.6 (q, SiC(CH3)3), 18.0 (s, SiC(CH3)3), -5.3, -5.1 (all q, Si(CH3)2).
IR (thin film) cm-1: 2988 w, 2955 s, 2934 s, 2893 w, 2859 w, 1381 m, 1372 s, 1255 s, 1217 s, 1132 s, 1078 s, 1022 s, 855 s, 779 m.
For C18H34O6Si (Mr = 374.55) Calcd.: 57.72 % C, 9.15 % H; Found: 57.51 % C, 9.18 % H.
3-O-[(tButyl)-dimethylsilyl]-1,2-O-isopropylidene-α-D-glucofuranose 5c
The glucofuranose bisacetonide (4c, 2.9 g, 7.7 mmol), dissolved in aq. acetic acid (75%, 30 mL), was stirred at room temperature for 48 h (TLC-monitoring). Removal of solvents in vacuo (20 mbar) leaves a yellow oil, which was dried in desiccator (NaOH, 20 mbar) for 2 days and purified by chromatography on silica gel 100g (eluent AcOEt - i-hexane 1:1). The furanose monoacetonide 5c was obtained as a colourless oil; yield 1.9 g (76%), Rf =0.3 (AcOEt - i-hexane 1:1), =-24.3 (c= 0.77, CHCl3).
Spectral Data
1H-NMR (CDCl3) δ: 5.82 (d, 1H, H-1, J1,2=3.6 Hz), 4.34 (d, 1H, H-2, J1,2=3.7 Hz), 4.29 (d, 1H, H-3, J3,4=2.7 Hz), 4.03 (dd, 1H, H-4, J3,4 =2.7 Hz, J4,5=8.2 Hz), 3.87 (ddd, 1H, H-5, dX of ABX, JA,X=5.3 Hz, JB,X=3.2 Hz, J4,5=8.2 Hz), 3.81 (dd, 1H, H-6, B of ABX, JA,B=11.5 Hz, JB,X=3.2 Hz), 3.73 (dd, 1H, H-6, A of ABX, JA,B=11.5 Hz, JA,X=5.3 Hz), 1.27, 1.46 (all s, 3H, C(CH3)3), 0.88 (s, 9H, SiC(CH3)3), 0.14, 0.12 (all s, 3H, Si(CH3)2).
13C-NMR (CDCl3) δ: 118.8 (s, C(CH3)2), 104.9 (d, C-1), 85.3, 80.8, 75.6, 64.4 (all d, C-2, C-3, C-4, C-5), 68.6 (t, C-6), 26.2, 26.7 (all q, C(CH3)2), 25.6 (q, SiC(CH3)3), 18.0 (s, SiC(CH3)3), -5.1, -4.9 (all q, Si(CH3)2).
IR (thin film) cm-1: 3436 br s ( OH), 2955 s, 2932 s, 2888 s, 2859 s, 1383 m, 1375 m, 1217 s, 1134 s, 1082 s, 1018 s, 835 s, 779 m.
For C15H30O6Si (Mr = 334.49) Calcd.: 53.86 % C, 9.04 % H; Found: 53.97 % C, 8.95 % H.
1,2:5,6-di-O-Isopropylidene-3-O-mesyl-α-D-glucofuranose 4d
Prepared from bisacetone D-glucose (
4a, 4 g, 15 mmol), mesylchloride (1.40 mL, 18 mmol, 1.2 equiv.) and pyridine (20 mL) under Ar atmosphere according to lit. [
21]. Yield 3.771 g (74%), m.p.= 78 – 80 °C, R
f = 0.54 (AcOEt -
i-hexane 1:1),
= -47.5 (c=0.53, CHCl
3), which was used without further purification {Lit. [
21]: m.p.=80 – 82 °C,
=- 49 -1 (c=1.0, CHCl
3)}.
Spectral Data
1H-NMR (CDCl3) δ: 5.88 (d, 1H, H-1, J1,2=3.6 Hz), 4.91 (d, 1H, H-3, J3,4=2.8 Hz), 4.72 (d, 1H, H-2, J1,2=3.6 Hz), 4.11 (m, 3H, H-4, H-5, H-6), 3.94 (dd, 1H, H-6, A of ABX, JA,B=8.9 Hz, JA,X=4.3 Hz), 3.03 (s, 3H, SO2CH3), 1.44, 1.36, 1.26, 1.25 (all s, 3H, CH3).
13C-NMR (CDCl3) δ: 112.7, 109.6 (all s, C(CH3)2), 105.2 (d, C-1), 83.7, 82.7, 79.8, 72.1 (all d, C-2, C-3, C-4, C-5), 67.6 (t, C-6), 38.0 (q, SO2CH3), 26.9, 26.6, 26.2, 25.2 (all s, CH3).
For C13H22O8S (Mr=338.38).
1,2-O-Isopropylidene-3-O-mesyl-α-D-glucofuranose 5d
Following the procedure described for preparation of
5c; the bisacetonide (
4d, 3.5 g,10.3 mmol) was stirred in aq. acetic acid (75%, 30 mL) at room temperature for 24 h (TLC - monitoring). The monoacetonide
5d was obtained as a yellow, but analytically pure oil; yield 2.93 g (95%), R
f=0.3 (AcOEt -
i-hexane 1:1),
=-21 (c=0.42, CHCl
3). {Lit. [
22]: [α]
D=-20.6 (c=1.0, CHCl
3)}.
Spectral Data
1H-NMR (CDCl3) δ: 5.93 (d, 1H, H-1, J1,2=3.7 Hz), 5.10 (d, 1H, H-3, J3,4=2.5 Hz), 4.76 (d, 1H, H-2, J1,2=3.7 Hz), 4.22 (dd, 1H, H-4, J3,4=2.5 Hz), 3.86 (bd, 2H, H-6, AB of ABX, JA,B=9.4 Hz), 3.73 (m, 1H, H-5, X of ABX), 3.14 (s, 3H, SO2CH3), 1.49, 1.39 (all s, 3H, CH3).
13C-NMR (CDCl3) δ: 112.7 (s, C(CH3)2), 105.0 (d, C-1), 83.3, 82.0, 78.4, 68.2 (all d, C-2, C-3, C-4, C-5), 63.9 (t, C-6), 38.1 (q, SO2CH3), 26.4, 26.1 (all s, CH3).
For C10H18O8S (Mr=298.31).
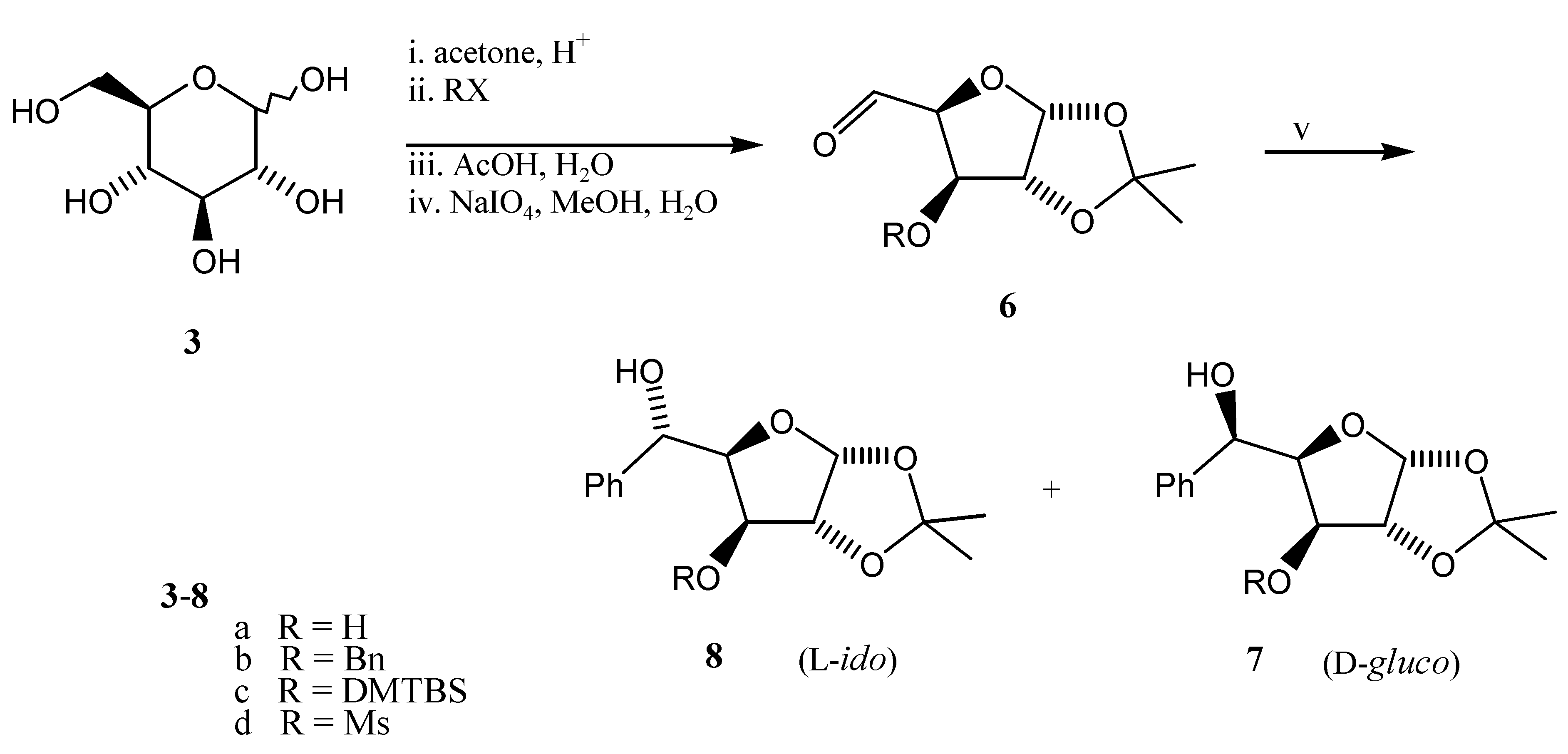
1,2-O-Isopropylidene-3-O-R-α-D-xylopentodialdo-1,4-furanose 6a-d
Prepared from monoacetone glucoses
4a-d by modified procedures of Inch [
6] and Lichtenthaler [
7]. To a cooled solution (0 °C) of
4 in water/methanol (1:2) was added in one portion NaIO
4 and stirring was continued at 0-20 °C for 2 h (TLC - monitoring). The solid precipitate ( NaIO
3) was filtered off and the solvent was removed on a rotavapory (20 mbar, 25 °C), the residue was partioned between chloroform (3 x 50 mL) and water (15 mL), dried ( Na
2SO
4), and concentrated at reduced pressure to a colourless oil, which was additionally dried in a desiccator (P
2O
5, 0.01 mbar) for 2 h. The crude products of diol - cleavage were used in the next reactions without another purification.
1,2-O-Isopropylidene-α-D-xylopentodialdo-1,4-furanose 4a
Prepared from 5a (1 g, 4.54 mmol) and NaIO4 (1.1 g, 5.14 mmol) in water/methanol (1:2, 20 mL). Yield 580 mg (68%), Rf=0.6 (AcOEt).
Spectral Data
1H-NMR (CDCl3) δ: 7.40 (bs, 1H, H-5), 5.98 (bs, 1H, H-1), 3.97-4.55 (m, 3H, H-2, H-3, H-4), 1.47, 1.31 (all s, 3H, CH3).
For C8H12O5 (Mr=188.18).
3-O-Benzyl-1,2-O-isopropylidene-α-D-xylopentodialdo-1,4-furanose 4b
Prepared from 5b (1.0 g, 3 mmol), NaIO4 (1.28 g, 6 mmol, 2 equiv) in water/methanol (1:2, 15 mL). Yield 803 mg (96%), Rf=0.6 (AcOEt- i-hexane 1:1).
Spectral Data
1H-NMR (CDCl3) δ: 9.68 (s, 1H, H-5), 7.26-7.35 (m, 5H, C6H5), 6.12 (s, 1H, H-1, J1,2=3.5 Hz), 4.63 (m, 4H, H-2, H-4, CH2), 4.32 (d, 1H, H-3, J3,4=3.1 Hz), 1.46, 1.40 (all s, 3H, CH3).
For C15H18O5 (Mr=278.31).
3-O-[(tButyl)-dimethylsilyl]-1,2-O-Isopropylidene-α-D-xylopentodialdo-1,4-furanose 6c
From 5c (1.69 g, 5.0 mmol), NaIO4 (2.13 g, 10 mmol, 2 equiv) in water/methanol (1:2, 30 mL). Yield 1.305 g (86%), Rf =0.68 (AcOEt).
For C14H26O5Si (Mr=302.44).
1,2-O-Isopropylidene-3-O-mesyl-α-D-xylopentodialdo-1,4-furanose 6d
From 5d (3.28 g, 11 mmol), NaIO4 (4.705 g, 22 mmol, 2 equiv.) in water/methanol (1:2, 30 mL). Yield 2.41 g (82%), Rf=0.27 (AcOEt - i-hexane 1:1).
For C9H14O7S (Mr=266.27).
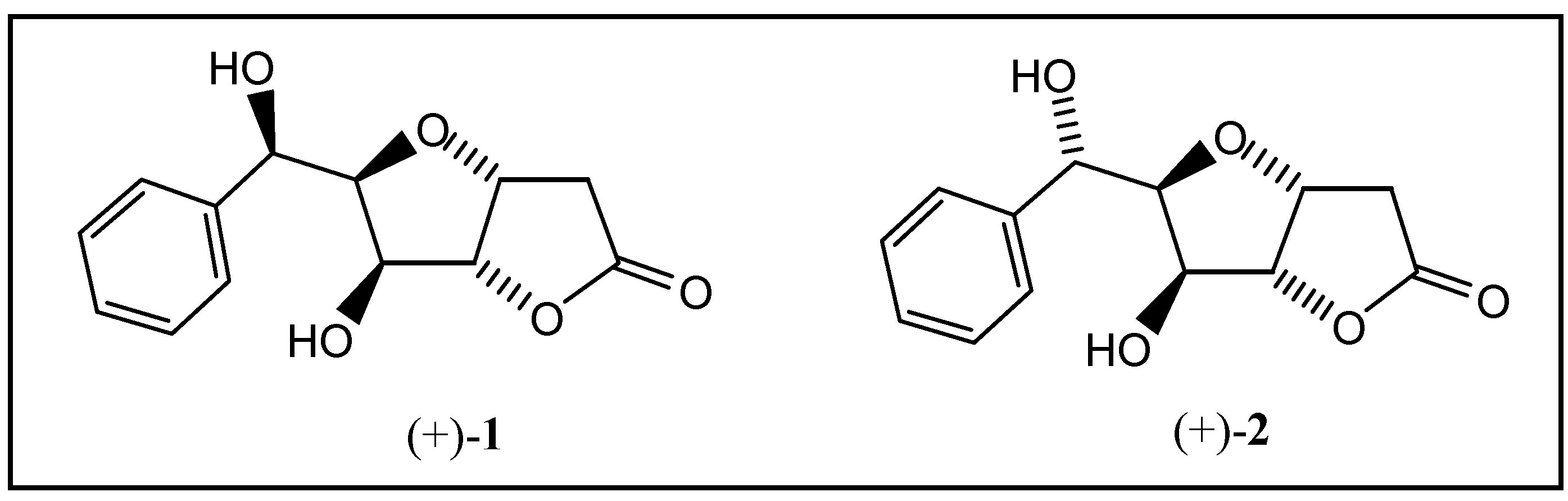
1,2-O-Isopropylidene-3-O-R-5-C-phenyl-β-L-ido- 8a-d and α-D-glucopentofuranose 7a-d
Prepared from aldehydes
6a-d according to lit. [
4,
6]. The crude aldehydes
6a-d were dissolved in dry ether and added dropwise to a solution of phenylmagnesium bromide in ether, prepared from bromobenzene and magnesium at –10 °C during 2 h. The mixtures were stirred at 0 °C for 4 h, and at r.t. for 24 h, then quenched with cold, saturated aqueous ammonium chloride and extracted with ether. After drying (Na
2SO
4) and solvent removal a yellow oils were obtained, whose were purified by flash chromatography on silica gel.
3-O-Benzyl-1,2-O-isopropylidene-5-C-phenyl-β-L-ido- 8b and α-D-glucopentofuranose 7b
Prepared from aldehyde
6b (1.113 g, 4 mmol), bromobenzene (1.05 mL, 10 mmol, 2.5 equiv) and magnesium (0.243 g, 10 mmol) in ether (35 mL). Yield 1.285 g (90%) of the mixture of diastereomers
8b and
7b (14:1) was purified by chromatography (silica gel, 40 g, AcOEt -
i-hexane 1:2); fraction 1 compound
7b (D-
gluco), 90 mg (6.3 %), yellow oil, R
f=0.35 (AcOEt -
i-hexane 1:2),
=-74 (c=1.0, CHCl
3). {Lit. [
12]:
=-77 (c=4.02, CHCl
3), lit. [
9]:
=-93 (CHCl
3), lit. [
6]:
=-76 (c=1.0, CHCl
3)}; fraction 2 compound
7b (L-
ido); 580 mg (41 %), colourless oil, R
f=0.26 (AcOEt -
i-hexane 1:2),
=-32.1 (c=0.76, CHCl
3), {lit. [
12]:
=-33.5 (c =2.0, CHCl
3)}. In addition, 330 mg (23 %) of an intermediate fraction containig both
7b and
8b was collected.
Alcohol 8b (L-ido)
Spectral Data
1H-NMR (CDCl3) δ: 7.31- 7.48 (m, 10H, C6H5), 6.03 (d, 1H, J1,2=3.8 Hz, H-1), 5.07 (d, 1H, J4,5=7.6 Hz, H-5), 4.61 (d, 1H, J1,2=3.8 Hz, H-2), 4.56 (d, 1H, J=11.5 Hz, CH2), 4.34 (dd, 1H, J3,4=3.2 Hz, J4,5=7.6 Hz, H-4), 4.32 (d, 1H, J=11.7 Hz, CH2), 3.64 (d, 1H, J3,4=3.1Hz, H-3), 1.50 , 1.32 (all s, 3H, C(CH3)2).
13C-NMR (CDCl3) δ: 139.7, 137.0 (all s, i-C6H5), 128.5, 128.3, 128.1, 128.0, 127.7, 127.1 (all d, C6H5), 111.9 (s, C(CH3)2), 105.2 (d, C-1), 71.8 (t, CH2), 72.4, 82.2, 82.2, 84.5 (all d, C-2, C-3, C-4, C-5), 26.3, 26.8 (all q, C(CH3)2).
IR (thin film)/ cm-1: 3453 m (OH), 2979 m, 2932 m, 2892 m, 1497 m, 1455 s, 1375 s, 1217 s, 1076 s, 1022 s, 760 s, 700 s.
For C21 H24O5 (Mr =356.42) Calcd.: 70.77 %C, 6.97 %H; Found: 71.09 %C, 6.91 %H.
Alcohol 7b (D-gluco)
Spectral Data
1H NMR (CDCl3) δ: 7.34- 7.43 (m, 10H, C6H5), 6.12 (d, 1H, J1,2=3.8 Hz, H-1), 5.23 (d, 1H, J4,5=6.1 Hz, H-5), 4.72 (d, 1H, J=11.6 Hz, CH2), 4.70 (d, 1H, J1,2=3.9 Hz, H-2), 4.51 (d, 1H, J=11.4 Hz, CH2), 4.45 (dd, 1H, J3,4=3.3 Hz, J4,5=6.1 Hz, H-4), 4.10 (d, 1H, J3,4=3.2Hz, H-3), 1.55, 1.38 (all s, 3H, C(CH3)2).
13C-NMR (CDCl3) δ: 141.8, 137.6 (all s, i-C6H5), 129.9, 129.7, 128.9, 128.8, 127.7, 127.2 (all d, C6H5), 113.1 (s, C(CH3)2), 106.2 (d, C-1), 73.4 (t, CH2), 73.4, 82.8, 83.5, 83.9 (all d, C-2, C-3, C-4, C-5), 27.3, 27.8 (all q, C(CH3)2).
IR (thin film)/ cm-1: 3450 m (OH), 2980 m, 2930 m, 1497 m, 1455 s, 1456 s, 1376 s, 1220 s, 1070 s, 1024 s, 760 s, 700 s.
For C21 H24O5 (Mr=356.42) Calcd.: 70.77 %C, 6.97 %H; Found: 71.12 %C, 6.93 %H.
3-O-[(tButyl)-dimethylsilyl]-1,2-O-isopropylidene-5-C-phenyl-β-L-ido- 8c and α-D-glucopento- furanose 7c
Prepared from 6c (1.305 g, 4.3 mmol), bromobenzene (1.37 mL, 13 mmol, 3 equiv) and magnesium (0.316 g, 13 mmol) in ether (40 mL). Yield 1.382 g (84 %), yellow oil, Rf=0.26 (AcOEt - i-hexane 1:3). The product consists of a 2:1 mixture of L-ido/D-gluco diastereomers 8c/7c by 1H-NMR. The NMR data are given from this mixture and probable assignments may eventually be reversed.
Spectral Data for alcohol 8c (L-ido)
1H-NMR (CDCl3) δ: 7.26- 7.36 (m, 5H, C6H5), 6.01 (d, 1H, J1,2=3.6 Hz, H-1), 4.95 (d, 1H, J4,5=5.8 Hz, H-5),4.38 (m, 2H, J1,2=3.5 Hz, J3,4=3.2 Hz, H-2, H-4), 4.12 (d, 1H, J3,4=2.9 Hz, H-3), 1.48, 1.31 (all s, 3H, C(CH3)2), 0.94 (s, 9H, SiC(CH3)3), 0.10, -0.04 (all s, Si(CH3)2).
13C-NMR (CDCl3) δ: 139.8 (s, i-C6H5), 128.4, 128.1, 127.3 (all d, C6H5), 111.9 (s, C(CH3)2), 104.8 (d, C-1), 72.4, 76.9, 83.9, 85.7 (all d, C-2, C-3, C-4, C-5), 26.4, 26.9 (all q, C(CH3)2) 25.7 (q, C(CH3)3), 17.9 (s, C(CH3)3), -4.4, -5.2 (all q, Si(CH3)2).
Spectral Data for alcohol 7c (D-gluco)
1H-NMR (CDCl3) δ: 7.26- 7.38 (m, 5H, C6H5), 5.94 (d, 1H, J1,2=3.8 Hz, H-1), 4.98 (m, 1H, H-5), 4.35 (m, 3H, H-2, H-3, H-4), 1.46 , 1.30 (all s, 3H, C(CH3)2), 0.94 (s, 9H, SiC(CH3)3), 0.16, 0.10 (all s, Si(CH3)2).
13C-NMR (CDCl3) δ: 141.7 (s, i-C6H5), 128.4, 127.8, 126.5 (all d, C6H5), 111.6 (s, C(CH3)2), 105.0 (d, C-1), 72.1, 76.3, 83.0, 85.3 (all d, C-2, C-3, C-4, C-5), 26.4, 26.9 (all q, C(CH3)2) 25.7 (q, C(CH3)3), 17.9 (s, C(CH3)3), -4.6, -5.1 (all q, Si(CH3)2).
IR (thin film)/ cm-1: 3439 br m (OH), 2955 s, 2932 s, 1383 w, 1375 w, 1254 m, 1219 m, 1165 m, 1132 m, 1078 s, 844 s, 835 s, 777 m, 764 m.
For C20 H32O5Si (Mr=356.42) Calcd.: 63.12 %C, 8.48 %H; Found: 63.39 %C, 8.46 %H.
1,2-O-Isopropylidene-3-O-mesyl-5-C-phenyl-β-L-ido- 8d and α-D-gluco-pentofuranose 7d
From 6d (1.602 g, 6 mmol), bromobenzene (1.9 mL, 18 mmol, 3 equiv) and magnesium (437 mg, 18 mmol) in ether (45 mL). Yield 1.612 g (78 %) as a yellow oil, Rf=0.28 (AcOEt - i-hexane 1:1). The product consists of a 1.6:1 mixture of L-ido/D-gluco diastereomers 8d/7d. The NMR data are given from this mixture and probable assignments may eventually be reversed.
Spectral Data for alcohol 8d (L-ido)
13C-NMR (CDCl3) δ: 133.7 (s, i-C6H5), 129.0, 128.6, 127.3 (all d, C6H5), 112.7 (s, C(CH3)2), 104.6 (d, C-1), 72.2, 81.7, 82.7, 83.6 (all d, C-2, C-3, C-4, C-5), 26.6, 26.3 (all q, C(CH3)2) 37.9 (q, SO2CH3).
Spectral Data for alcohol 7d (D-gluco)
13C-NMR (CDCl3) δ: 138.4 (s, i-C6H5), 129.3, 128.9, 127.5 (all d, C6H5), 111.9 (s, C(CH3)2), 105.0 (d, C-1), 73.4, 75.3, 82.2, 85.1 (all s, C-2, C-3, C-4, C-5), 26.7, 26.1 (all q, C(CH3)2) 37.9 (q, SO2CH3).
IR (thin film)/ cm-1: 3486 br m (OH), 2990 m, 2936 m, 1363 s (SO), 1341 s, 1306 s, 1170 s (SO), 1163 w, 1152 s, 1088 s, 1024 s, 972 s, 957 s, 909 s, 845 s, 835 s.
For C15 H20O7S (Mr=344.39) Calcd.: 52.31 %C, 5.85 %H; Found: 52.21 %C, 5.94 %H.
3-O-Benzyl-1,2-O-isopropylidene-5-C-phenyl-α-D-gluco 7b and β-L-ido-pentofuranose 8b
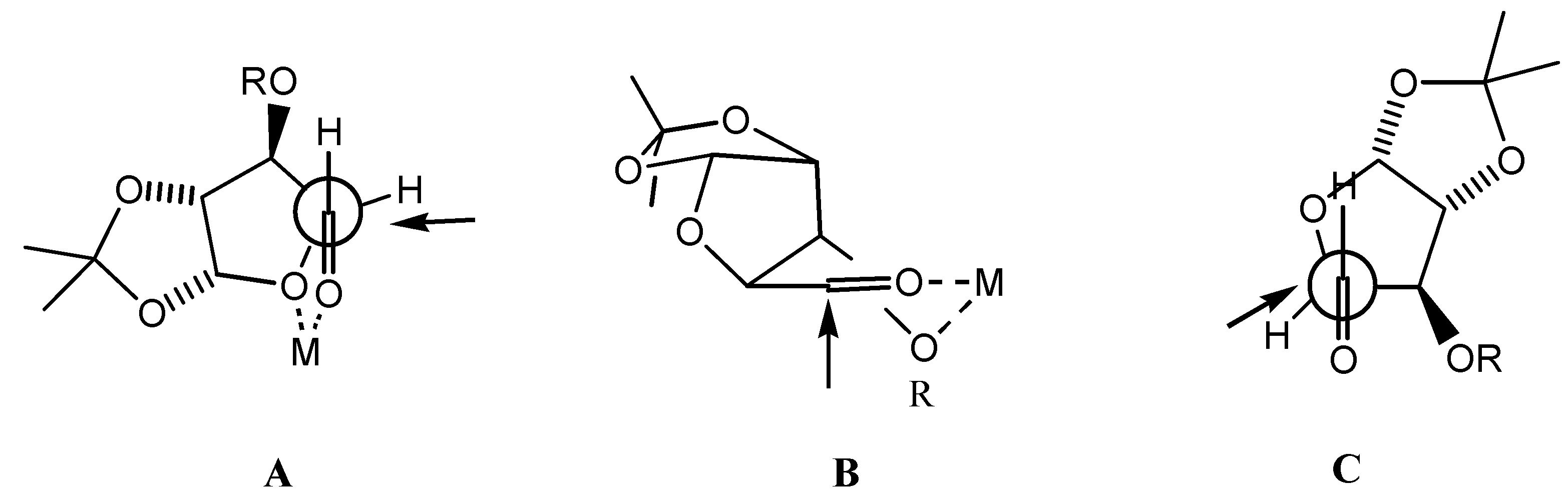
To a freshly prepared solution of phenylmagnesium bromide [prepared from bromobenzene (0.8 mL, 7.6 mmol, 2.5 equiv) and magnesium (0.185 g, 7.6 mmol) in dry ether (15 mL) by standard procedure] a solution of 18-C-6 (2.009 g, 7.6 mmol, 2.5 equiv) and dibenzo 18-C-6 crown-ether (2.739 g, 7.6 mmol) in ether (10 mL) was added dropwise at room temperature. The mixture was additionally treated in ultrasound bath for 30 min. Then the aldehyde 6b (865 mg, 3 mmol) was added at –10 °C. The mixture was stirred at 0 °C for 4 h, and at r.t. for 24 h, then quenched with saturated aqueous ammonium chloride (30 mL) and extracted with ether. After drying (Na2SO4) and solvent removal a yellow oil was obtained; yield 950 mg (89 %) resp. 920 mg (86 %) as a 1.2:1 mixture of D-gluco/L-ido 7b/8b diastereomers.
To a stirred solution of aldehyde 6b (1.0 g, 3.59 mmol) in dry ether (20 mL) was added a solution of PhTi(OiPr)3 (1.195 g, 3.95 mmol, 1.1 equiv) in ether (20 mL) at –30 °C during 20 min. The mixture was stirred at –30 °C for 4 h and at r.t. for 48 h, then quenched with saturated aq. ammonium chloride (20 mL), extracted with ether (3 x 20 mL), dried with Na2SO4. After removal of solvent a yellow oil was obtained. Yield 383 mg (30 %). The product consists of a 14:1 mixture of 7b/ 8b diastereomers according to 1H-NMR data.
1,2-O-Isopropylidene-5-C-phenyl-α-D-gluco 7a and β-L-idopentofuranose 8a
Anhydrous cerium chloride (740 mg, 3 mmol) prepared by heating of CeCl
3.7 H
2O (1.12 g, 3 mmol) at 130-140 °C for 5 h
in vacuo [
23], was suspended in tetrahydrofuran (10 mL) at room temperature. The suspension was sonicated for 1 h, then stirred for 2 h at r.t. A solution of phenyllithium (3 mL, 1M solution in ether) was added at –10 °C and stirred for 45 min. A solution of crude
6a (580 mg, 3 mmol) in ether (5 mL) at –5 °C was added and stirring was continued at r.t. for 48 h, then quenched with saturated aqueous NaF (25 mL), extracted with ether (3 x 20 mL), dried with Na
2SO
4. After removal of solvent a yellow oil was obtained. Yield 544 mg (68 %). The product consists of a 1.6:1 mixture of D-
gluco/L-
ido diastereomers
7a/
8a according to
1H-NMR data.
Alcohol 8a (L-ido)
Spectral Data
1H-NMR (CD3OD) δ: 7.31- 7.57 (m, 5H, C6H5), 6.01 (d, 1H, J1,2=3.7 Hz, H-1), 4.98 (d, 1H, J4,5=8.4 Hz, H-5), 4.46 (dd, 1H, J1,2=3.7 Hz, J2,3=0.8 Hz, H-2), 4.24 (dd, 1H, J3,4=2.8 Hz, J4,5=8.4 Hz, H-4), 3.61 (bd, 1H, J3,4=3.1Hz, H-3), 1.49 , 1.31 (all s, 3H, C(CH3)2).
13C-NMR (CD3OD) δ: 143.4 (s, i-C6H5), 130.2, 129.9, 129.2 (all d, C6H5), 113.7 (s, C(CH3)2), 107.4 (d, C-1), 79.6, 76.6, 86.9, 87.8 (all d, C-2, C-3, C-4, C-5), 28.0, 27.4 (all q, C(CH3)2).
Alcohol 7a (D-gluco)
Spectral Data
1H-NMR (CDCl3) δ: 7.34- 7.43 (m, 10H, C6H5), 6.12 (d, 1H, J1,2=3.8 Hz, H-1), 5.23 (d, 1H, J4,5=6.1 Hz, H-5), 4.72 (d, 1H, J=11.6 Hz, CH2), 4.70 (d, 1H, J1,2=3.9 Hz, H-2), 4.51 (d, 1H, J=11.4 Hz, CH2), 4.45 (dd, 1H, J3,4=3.3 Hz, J4,5=6.1 Hz, H-4), 4.10 (d, 1H, J3,4=3.2Hz, H-3), 1.55, 1.38 (all s, 3H, C(CH3)2).
13C-NMR (CD3OD) δ: 144.6 (s, i-C6H5), 130.2, 129.6, 129.0 (all d, C6H5), 113.6 (s, C(CH3)2), 107.2 (d, C-1), 73.1, 76.5, 85.6, 87.4 (all d, C-2, C-3, C-4, C-5), 28.0, 27.4 (all q, C(CH3)2).