Synthesis and Antimicrobial Activity of Some New Benzimidazole Derivatives
Abstract
:Introduction
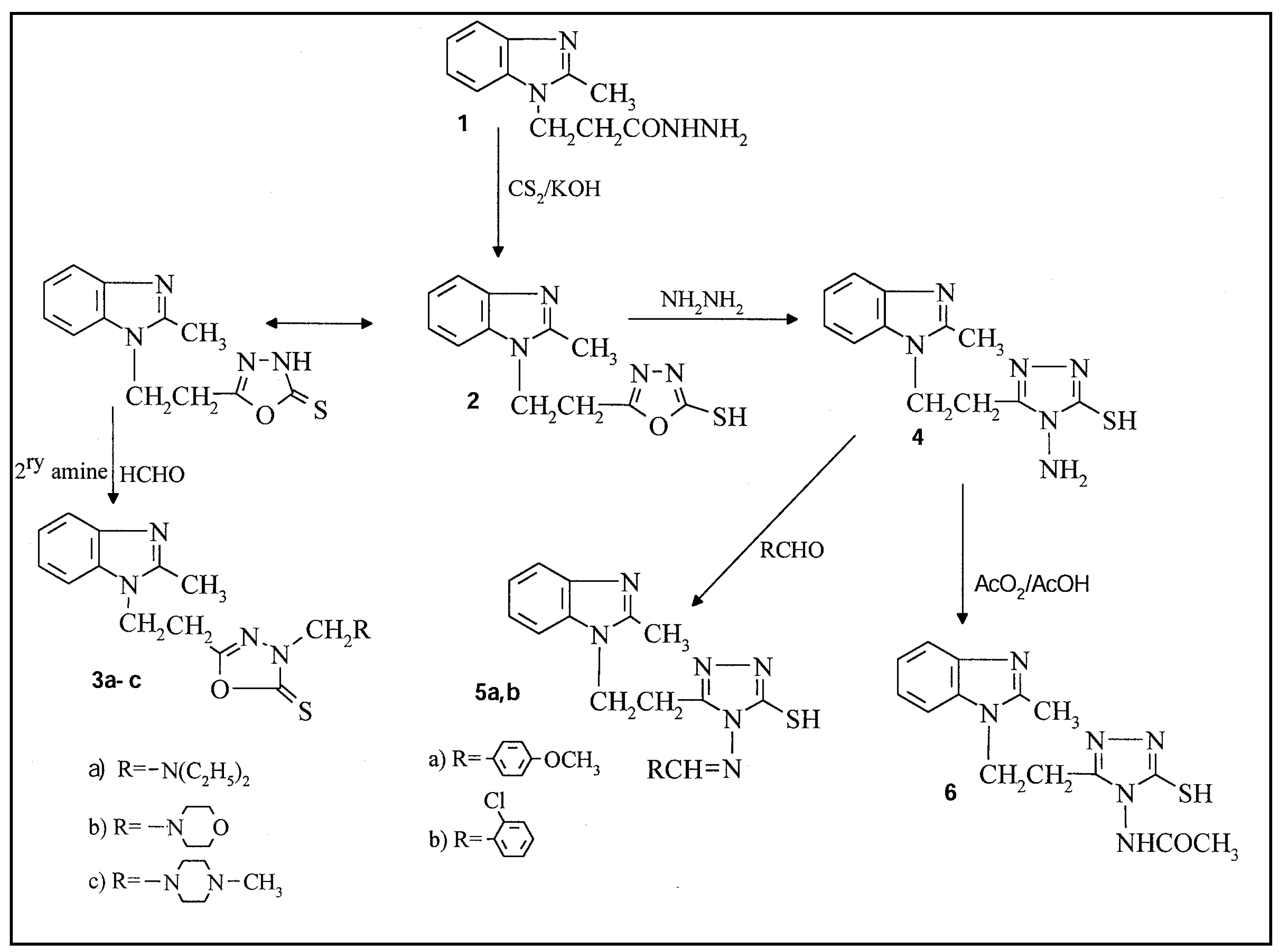
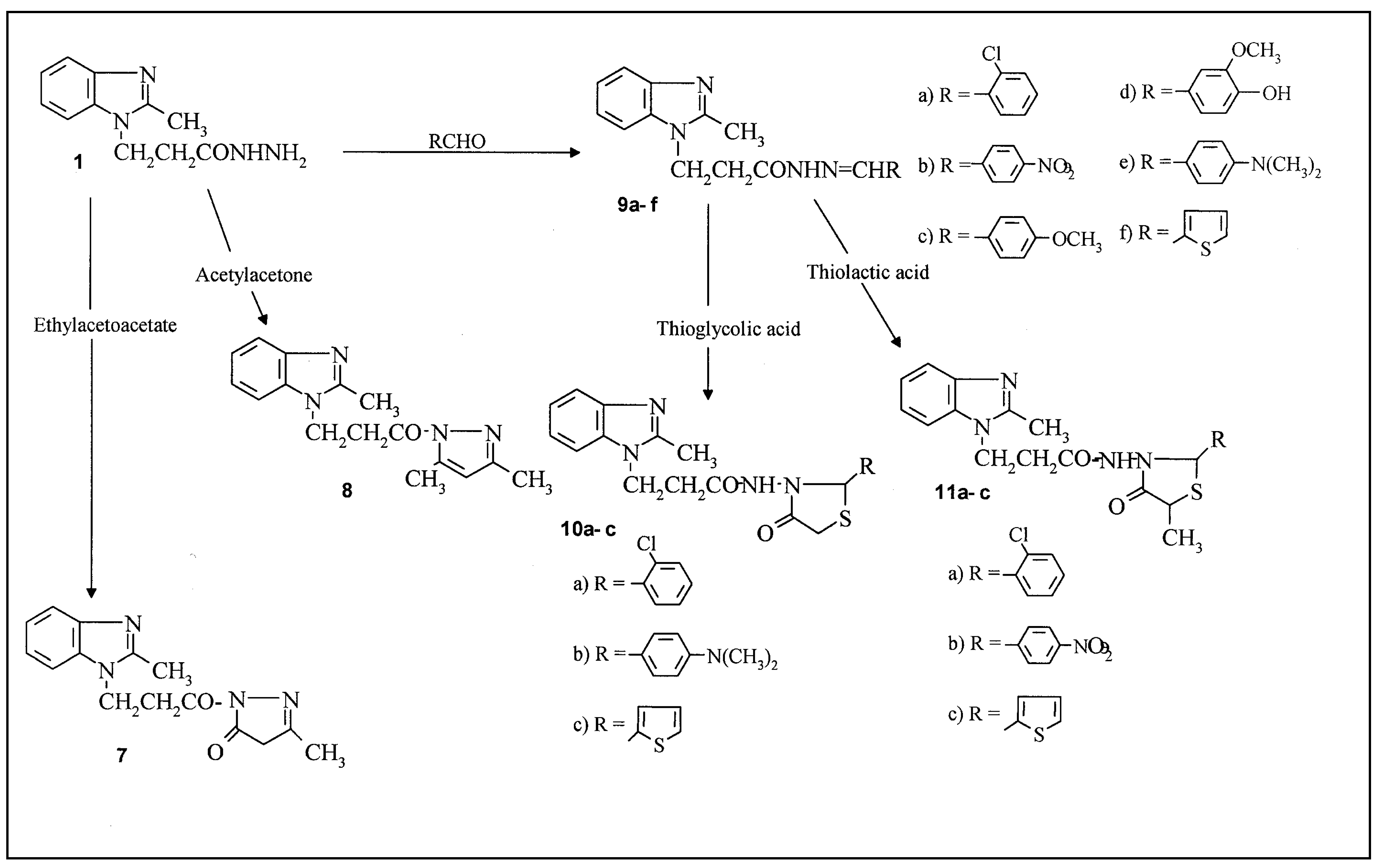
Results and Discussion
Biological Screening. Antimicrobial activity test
| Compound No. | B. cereus . | E. coli . | S. cerevisae . | A. niger . |
|---|---|---|---|---|
| Gentamycine | +++ | +++ | - | - |
| Ampicelline | +++ | +++ | - | - |
| 1 | - | - | - | - |
| 2 | +++ | + | - | - |
| 3a | + | - | - | - |
| 4 | ++ | - | - | - |
| 5b | - | - | - | - |
| 8 | - | - | - | - |
| 9a | ++ | - | - | - |
| 10a | - | - | - | - |
| 10c | + | - | - | - |
| 11a | - | - | - | - |
| 11c | - | + | - | - |
- Highly active = +++ (inhibition zone > 12 mm)
- Moderately active = ++ (inhibition zone 9 - 12 mm)
- Slightly active = + (inhibition zone 6 - 9 mm)
- Inactive = - (inhibition zone < 6 mm)
Experimental
Materials and Methods
Synthesis of compounds
5-[2-(2-Methylbenzimidazol-1-yl) ethyl][1,3,4]oxadiazole-2(3H)-thione (2)
5-[2-(2-Methylbenzimidazol-1-yl) ethyl]-3-diethylaminomethyl (or 3-N-morpholinylmethyl, or 3-N-piprazinylmethyl)[1,3,4]oxadiazole-2(3H)-thione (3a-c).
General method
5-[2-(2-Methylbenzimidazol-1-yl) ethyl]-4-amino[1,2,4]triazole-3-thiol (4)
5-[2-(2-Methylbenzimidazol-1-yl)ethyl]-4-arylidenimino-[1,2,4]triazole-3-thiol (5a,b)
General method
5-[2-(2-Methylbenzimidazol-1-yl)ethyl]-4-actamido-[1,2,4]triazole-3-thiol (6)
1-[3-(2-Methylbenzimidazol-1-yl)propanoyl]-4,5-dihydro-3-methylpyrazol-5-one (7)
1-[3-(2-Methylbenzimidazol-1-yl)propanoyl]-3,5-dimethylpyrazole (8)
3-(2-Methylbenzimidazol-1-yl)propanoic acid hydrazide Schiff’s bases (9a-f )
General method
3-(2-Methylbenzimidazol-1-yl)-N-(2-aryl-4-oxo[1,3]thiazolidin-3-yl)propionamide (10a-c)
General method
3-(2-Methylbenzimidazol-1-yl)-N-(2-aryl-5-methyl-4-oxo[1,3]thiazolidin-3-yl)propionamide (11a-c)
General method
| Compd. No | Spectral data |
|---|---|
| 2 | IR (KBr, cm-1): 3300 (NH), 2923 (CH), 2600 (SH), 1624 (C=N) and 1136.0 (C=S), [(SH) and (C=S) absorptions indicate thiol-thione tautomers]. 1H-NMR (DMSO-d6, δ ppm): 2.55 (3H, s, CH3 at C-2 of benzimidazole), 3.25 (2H, t, J = 7 Hz, NCH2CH2), 4.6 (2H, t, J = 7 Hz, NCH2CH2) and 7.25-7.6 (5H, m, aromatic protons, and SH). MS, m/z (%): 260 (M+, 57.72), 145 (100). |
| 3a | IR (KBr, cm-1): 2991 (CH), 1180 (C-O), 1654 (C=N) and 1150 (C=S). 1H-NMR (CDCl3, δ ppm): 1.2 (6H, t, J = 7 Hz, 2 CH2CH3), 2.55 (3H, s, CH3 at C-2 of benzimidazole), 2.75 (2H, t, J = 7 Hz, NCH2CH2), 3.6 (4H, q, J = 6 Hz, 2 CH2 CH3), 4.45 (2H, t, J = 7 Hz, NCH2CH2), 4.9 (2H, s, NCH2N) and 7.15-7.6 (4H, m, aromatic protons). |
| 3b | IR: (KBr, cm-1), 2985 (CH), 1177 (C-O), 1660 (C=N) and 1147 (C=S). 1H-NMR (CDCl3, δ ppm): 2.55 (4H, t, J = 7 Hz, N(CH2)2 of morpholine ring), 2.6 (3H, s, CH3 at C-2 of benzimidazole), 3.2 (2H, t, NCH2CH2), 3.65 (4H, t, J = 7 Hz, O(CH2)2 of morpholine ring), 4.5 (2H, t, J = 7 Hz, NCH2CH2) 4.85 (2H, s, NCH2N) and 7.15-7.7 (4H, m, aromatic protons). |
| 3c | IR (KBr, cm-1): 2988 (CH), 1176 (C-O), 1658 (C=N) and 1148 (C=S). MS: (M+.,C18H24N6OS), m/z 372.5 (0.09, unstable) and the base peak at m/z 145 (100). |
| 4 | IR (KBr, cm-1): 3420, 3300, 3145 (NH2 sym., asym-stretch and NH), 2600 (SH), 1155 (C=S) (thiol-thionetautomers) and 1620 (C=N). 1H-NMR (DMSO-d6, δ ppm): 2.5 (3H, s, CH3 at C-2 of benzimidazole), 3.2 (2H, t, J = 7 Hz, NCH2CH2), 4.55 (2H, t, J = 7 Hz, NCH2CH2 ), 5.6 (2H, s, NH2) and 7.25-7.6 (5H, m, aromatic protons and SH). MS, m/z (%):274 (M+ , 76.46), 145 (100). |
| 5a | IR (KBr, cm-1): 3047 (NH), 2993 (CH), 2580 (SH), 1267 (C=S) and 1153 (C-O). 1H-NMR (DMSO-d6, δ ppm): 2.5 (3H, s, CH3 at C-2 of benzimidazole), 3.2 (2H, t, J = 7 Hz, NCH2CH2), 3.85 (3H, s, OCH3), 4.55 (2H, t, J = 7 Hz, NCH2CH2) and 7.1-7.8 (8H, m, aromatic protons), 9.4 (1H, s, CH=N) and 13.8 (1H, s, NH). MS, m/z (%): 392 (M+ , 14.05), 132 (100). |
| 5b | IR (KBr, cm-1): 3162 (NH), 2986 (CH), 2593 (SH), 1282 (C=S) and 746 (C-Cl). MS, m/z (%): 398 (M+, 37Cl, 0.29), 396 (M+, 35Cl, 0.60), 132 (100). |
| 6 | IR (KBr, cm-1): 3157 (NH), 2964 (CH), 2486 (SH) and 1697 (C=O). MS, m/z (%): 316 (M+ , 16.1), 145 (100). |
| 7 | IR (KBr, cm-1): 3210 (NH), 3005, 2995 (CH), 1732 (C=O, of pyrazolone), 1682 (C=O) and 1663 (C=N). 1H-NMR (CDCl3, δ ppm): 2.2 (3H, s, CH3 at C-3 of pyrazolone), 2.5 (3H, s, CH3 at C-2 of benzimidazole), 2.75 (2H, t, J = 7 Hz, NCH2CH2), 3.1 (2H, s, CH2 of pyrazolone), 4.5 (2H, t, J = 7 Hz, NCH2CH2), and 7.15-7.6 (4H, m, aromatic protons). MS, m/z (%): 284 (M+ , 0.33), 145 (100). |
| 8 | IR (KBr, cm-1): 2990 (CH) and 1668 (C=O). 1H-NMR (CDCl3, δ ppm): 1.55, 1.75 (6H, 2s, 2CH3 of pyrazoline), 2.5 (3H, s, CH3 at C-2 of benzimidazole), 2.95 (2H, t, J = 7 Hz,NCH2CH2), 4.45 (2H, t, J = 7 Hz, NCH2CH2) and 7.0-7.6 (5H, m, 4 aromatic protons and 1H of pyrazoline). |
| 9a | IR (KBr, cm-1): 3100 (NH), 2990 (CH), 1671 (C=O). MS, m/z (%): 342 (M+, 37Cl, 20.7), 340 (M+, 35Cl, 58.3), 145 (100). |
| 9b | IR (KBr, cm-1): 3045 (NH), 2964 (CH), 1671 (C=O), 1514, 1327 (NO2). |
| 9c | IR (KBr, cm-1): 3190 (NH), 3000 (CH), 1670 (C=O). 1H-NMR (CDCl3, δ ppm): 2.45 (3H, s, CH3 at C-2 of benzimidazole), 3.0 (2H, t, J = 7 Hz, NCH2CH2), 3.6 (3H, s, OCH3), 4.35 (2H, t, J = 7 Hz, NCH2CH2), 6.65-7.7 (9H, m, 8 aromatic protons and 1H of N=CH) and 10.3 (1H, s, NH). MS, m/z (%): 336 (M+ , 100). |
| 9d | IR (KBr, cm-1): 3400 (OH), 3200 (NH), 2968(CH), 1664 (C=O). 1H-NMR (DMSO-d6 , δ ppm): 2.5 (3H, s, CH3 at C-2 of benzimidazole), 3.0 (2H, t, J = 7 Hz, NCH2CH2), 3.7 (3H, s, OCH3), 4.35 (2H, t, J = 7 Hz, NCH2CH2), 6.65-7.9 (8H, m, 7 aromatic protons and 1H of N=CH), 9.5 (1H, s, OH) and 11.1 (1H, s, NH). MS, m/z (%): 352 (M+ , 57), 145 (100). |
| 9e | IR (KBr, cm-1): 3251 (NH), 2984 (CH), 1672 (C=O). 1H-NMR (DMSO-d6 , δ ppm): 2.5 (3H, s, CH3 at C-2 of benzimidazole), 2.95 (6H, s, N(CH3)2), 3.2 (2H, t, J = 7 Hz, NCH2CH2), 4.45 (2H, t, J = 7 Hz, NCH2CH2), 6.7-7.9 (9H, m, 8 aromatic protons and 1H of N=CH) and 11.1 (1H, s, NH). |
| 9f | IR (KBr, cm-1): 3209 (NH), 2997 (CH), 1668 (C=O). |
| 10a | IR (KBr, cm-1): 3417 (NH), 2930 (CH), 1716 (C=O of thiazolidinone), 1673 (C=O amide) and 746 (C-Cl). 1H-NMR (DMSO-d6, δ ppm): 2.5 (3H, s, CH3 at C-2 of benzimidazole), 2.65 (2H, t, J = 7 Hz, NCH2CH2), 3.75 (1H, d, J = 6 Hz, A part of an AB system, equatorial proton of thiazolidinone at C-5), 3.9 (1H, d, J = 6 Hz, B part of an AB system, axial proton of thiazolidinone at C-5), 4.35 (2H, t, NCH2CH2), 6.00 (1H, s,CH of thiazolidinone at C-2 ), 7.1-7.5 (8H, m, aromatic protons) and 10.4 (1H, s, NH). MS, m/z (%): 416 (M+, 37Cl, 3.62), 414 (M+, 35Cl, 10.70), 132 (100). |
| 10b | IR (KBr, cm-1): 3415 (NH), 2951 (CH), 1719 (C=O of thiazolidinone), 1676 (C=O amide). |
| 10c | IR (KBr, cm-1): 3418 (NH), 2930 (CH), 1720 (C=O of thiazolidinone), 1677 (C=O amide). 1H-NMR (DMSO-d6, δ ppm): 2.45 (3H, s, CH3 at C-2 of benzimidazole), 2.65 (2H, t, J = 7 Hz, NCH2CH2), 3.7 (1H, d, J = 6 Hz, A part of an AB system, equatorial proton of thiazolidinone at C-5), 4.0 (1H, d, J = 6 Hz, B part of an AB system, axial proton of thiazolidinone at C-5), 4.45 (2H, t, NCH2CH2), 5.95 (1H, s,CH of thiazolidinone at C-2 ), 6.7-7.5 (7H, m, aromatic protons) and 10.5 (1H, s, NH). MS, m/z (%): 386 (M+, 0.7), 132 (100). |
| 11a | IR (KBr, cm-1): 3400 (NH), 3000 (CH), 1715 (C=O of thiazolidinone), 1670 (C=O amide). 1H-NMR (CDCl3, δ ppm): 1.45 (3H, d, J = 6 Hz, CH3 at C-5 of thiazolidinone), 2.45 (3H, s, CH3 at C-2 of benzimidazole), 2.65 (2H, t, J = 7 Hz, NCH2CH2), 3.65 (1H, q, CH of thiazolidinone at C-5), 4.35 (2H, t, J = 7 Hz, NCH2CH2), 6.1 (1H, s, CH of thiazolidinone at C-2), 7.1-7.55 (8H, m, aromatic protons) and 10.4 (1H, s, NH). MS, m/z (%): 430 (M+, 37Cl, 8.89), 428 (M+, 35Cl, 25.78), 160 (100). |
| 11b | IR (KBr, cm-1): 3440 (NH), 2930 (CH), 1720 (C=O of thiazolidinone), 1677 (C=O amide) and 1520, 1325 (NO2). |
| 11c | IR (KBr, cm-1): 3341 (NH), 3008 (CH), 1711 (C=O of thiazolidinone), 1675 (C=O amide). 1H-NMR (DMSO-d6, δ ppm): 1.43 (3H, d, J = 6 Hz, CH3 at C-5 of thiazolidinone), 2.42 (3H, s, CH3 at C-2 of benzimidazole), 2.65 (2H, t, J = 7 Hz, NCH2CH2), 3.65 (1H, q, CH of thiazolidinone at C-5), 4.4 (2H, t, J = 7 Hz, NCH2CH2), 6.2 (1H, s, CH of thiazolidinone at C-2), 6.9-7.5 (7H, m, aromatic protons) and 10.4 (1H, s, NH). MS, m/z (%): 400 (M+, 0.6), 132 (100). |
| Comp No. | M.P. °C (Recryst. Solvents) | Yield % | Formulae (M.Wt.) | Analysis % ,Calculated / Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 2 | 208-210 (AcOH/H2O) | 86 | C12H12N4OS (260.28) | 55.37 55.29 | 4.65 4.63 | 21.52 21.45 | 12.32 12.28 |
| 3a | 89-91 (CHCl3/Pet.ether) | 65 | C17H23N5OS (345.51) | 59.10 59.18 | 6.71 6.77 | 20.27 20.32 | - |
| 3b | 183-185 (CHCl3/Pet.ether) | 60 | C17H21N5O2S (359.49) | 56.80 56.85 | 5.89 5.93 | 19.48 19.51 | - |
| 3c | 149-150 (CHCl3/Pet.ether) | 63 | C18H24N6OS (372.54) | 58.04 58.15 | 6.49 6.51 | 22.56 22.62 | - |
| 4 | 304-306 (AcOH/H2O) | 85 | C12H14N6S (274.35) | 52.53 52.45 | 5.14 5.12 | 30.63 30.54 | 11.68 11.65 |
| 5a | 261-263 (AcOH/H2O) | 80 | C20H20N6OS (392.48) | 61.20 61.27 | 5.15 5.17 | 21.42 21.43 | - |
| 5b | 249-251 (AcOH/H2O) | 85 | C19H17ClN6S (396.93) | 57.49 57.53 | 4.32 4.37 | 21.18 21.21 | - |
| 6 | 282-284 (Ethanol) | 68 | C14H16N6OS (316.43) | 53.14 53.09 | 5.11 5.20 | 26.56 26.54 | - |
| 7 | 123-124 (Ethanol) | 65 | C15 H16 N4 O2 (284.35) | 63.36 63.32 | 5.68 5.67 | 19.71 19.69 | - |
| 8 | 139-141 (Ethanol) | 75 | C16 H18 N4 O (282.38) | 68.06 68.09 | 6.43 6.47 | 19.85 19.90 | - |
| 9a | 187-189 (Ethanol/H2O) | 87.5 | C18H17ClN4O (340.84) | 63.43 63.41 | 5.04 5.01 | 16.44 16.40 | - |
| 9b | 199-201 (Ethanol/H2O) | 82.6 | C18H17N5O3 (351.36) | 61.53 61.59 | 4.88 4.82 | 19.93 19.99 | - |
| 9c | 167-169 (Ethanol/H2O) | 88 | C19H20N4O2 (336.43) | 67.84 67.87 | 6.00 6.12 | 16.66 16.69 | - |
| 9d | 229-231 (Ethanol/H2O) | 90 | C19H20N4O3 (352.43) | 64.74 64.71 | 5.73 5.69 | 15.90 15.96 | - |
| 9e | 179-181 (Ethanol/H2O) | 91 | C20H23N5O (349.48) | 68.73 68.68 | 6.65 6.60 | 20.04 20.12 | - |
| 9f | 89-91 (Ethanol/H2O) | 85 | C16H16N4OS (312.42) | 61.51 61.56 | 5.17 5.20 | 17.93 17.99 | - |
| 10a | 249-251 (Ethanol/H2O) | 71 | C20H19ClN4O2S (414.94) | 57.89 57.84 | 4.62 4.65 | 13.51 13.48 | 7.73 7.69 |
| 10b | 264-266 (Ethanol/H2O) | 64 | C22H25N5O2S (423.58) | 62.39 62.45 | 5.96 5.98 | 16.54 16.58 | 7.55 7.60 |
| 10c | 120-122 (Ethanol/H2O) | 40 | C18H18N4O2S2 (386.52) | 55.94 55.98 | 4.70 4.69 | 14.49 14.46 | 16.59 16.55 |
| 11a | 193-195 (CHCl3/Pet.ether) | 74 | C21H21ClN4O2S (428.97) | 58.80 58.87 | 4.94 4.96 | 13.06 13.11 | 7.47 7.50 |
| 11b | 159-161 (Ethanol/H2O) | 53 | C21H21N5O4S (439.53) | 57.38 57.35 | 4.83 4.82 | 15.94 15.91 | 7.29 7.25 |
| 11c | 131-133 (CHCl3/Pet.ether) | 44 | C19H20N4O2S2 (400.5) | 56.97 57.12 | 5.04 5.11 | 13.99 14.13 | 16.00 16.10 |
Acknowledgements
References
- Boruah, C. R.; Skibo, E. B. J. Med. Chem. 1994, 37, 1625. [PubMed]
- Srivastava, R. P.; Sharma, S. Die Pharmazie 1990, 45, 34. [PubMed]
- Kubo, K.; Inoda, Y.; Kohara, Y.; Sugiura, Y.; Ojima, M.; Itoh, K.; Furukawa, Y.; Nishikawa, K.; Naka, T. J. Med. Chem. 1993, 36, 1772. [PubMed]
- Kubo, K.; Kohara, Y.; Imamiya, E.; Sugiura, Y.; Inada, Y.; Furukawa, Y.; Nishikawa, K.; Naka, T. J. Med. Chem. 1993, 36, 2182. [PubMed]
- Kubo, K.; Kohara, Y.; Yoshmora, Y.; Inada; Shibouta, Y.; Furukawa, Y.; Kato, T.; Nishikawa, K.; Naka, T. J. Med. Chem. 1993, 36, 2343. [PubMed]
- Ito, K.; Kagawa, H.; Fududa, T.; Yoshino, K.; Nose, T. Arzneim-Forsch. 1982, 31, 49–55.
- Rizk, M. Egypt. J. Pharm. Sci. 1993, 34, 243.
- Kassem Emad, M. M. El-masry, Afaf. In Proceedings of The First International Scientific Conference (Science and Deveopment), Cairo, 20-23 March 1995; p. 119.
- Kamel, M. M.; Naser, M. E. Die Pharmazie 1979, 34(7), 440.
- Chiyomara, E. Y.; Dohke, G. Japan Pat. 460, 7339, (1973). Chem. Abstr., (1974), 81, 73392.
- Greenfield, S. A.; Michael, C. S.; Von Meyer, W. C.; (Rohm and Hass Co.). Ger. Offen. 1, 966,806, 1974. Chem. Abstr. 1975, 82, 150485.
- Popp, F. D. J. Org. Chem. 1961, 26, 1566.
- Popp, F. D. J. Med. Chem. 1964, 7, 210. [PubMed]
- Clinton, R. O.; Manson, A. J.; Stonner, F. W.; Beyler, A. L.; Potts, G. O. J. Amer. Chem. Soc. 1959, 81, 1513.
- Hassan, Abdelwahed Sameh. M. Sc.Thesis, Faculty of Science, Cairo University, 1999.
- Young, R. W.; Wood, K. H. J. Am. Chem. Soc. 1955, 77, 400.
- Burckhalter, J. H.; Wells, J. N.; Mayer, W. J. Tetrahedron Letts. 1964, 1353.
- Kamdar, G. C.; Chavda, A. C.; Parikh, A. R. J. Indian Chem. Soc. 1987, 64, 298.
- El-Bannany, A. A.; Abdel-Azim, H. I. Pharmazie 1986, 41, 144.
- Abou-Zeid, Abou-Zeid A.; Shehata, Youssef. Indian J. Pharm. 1969, 31(3), 72.
- Samples Availability: Available from the authors.
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
El-masry, A.H.; Fahmy, H.H.; Ali Abdelwahed, S.H. Synthesis and Antimicrobial Activity of Some New Benzimidazole Derivatives. Molecules 2000, 5, 1429-1438. https://doi.org/10.3390/51201429
El-masry AH, Fahmy HH, Ali Abdelwahed SH. Synthesis and Antimicrobial Activity of Some New Benzimidazole Derivatives. Molecules. 2000; 5(12):1429-1438. https://doi.org/10.3390/51201429
Chicago/Turabian StyleEl-masry, Afaf H., H. H. Fahmy, and S. H. Ali Abdelwahed. 2000. "Synthesis and Antimicrobial Activity of Some New Benzimidazole Derivatives" Molecules 5, no. 12: 1429-1438. https://doi.org/10.3390/51201429




