Cyclohexenonic Long-Chain Fatty Alcohols as Neuronal Growth Stimulators
Abstract
:- 1. Introduction
- 2. Neurotrophic compounds as therapeutic agents for neurodegenerative diseases
- 2.1. Natural neurotrophic factors
- 2.2. Non-peptide neurotrophic factor analogues
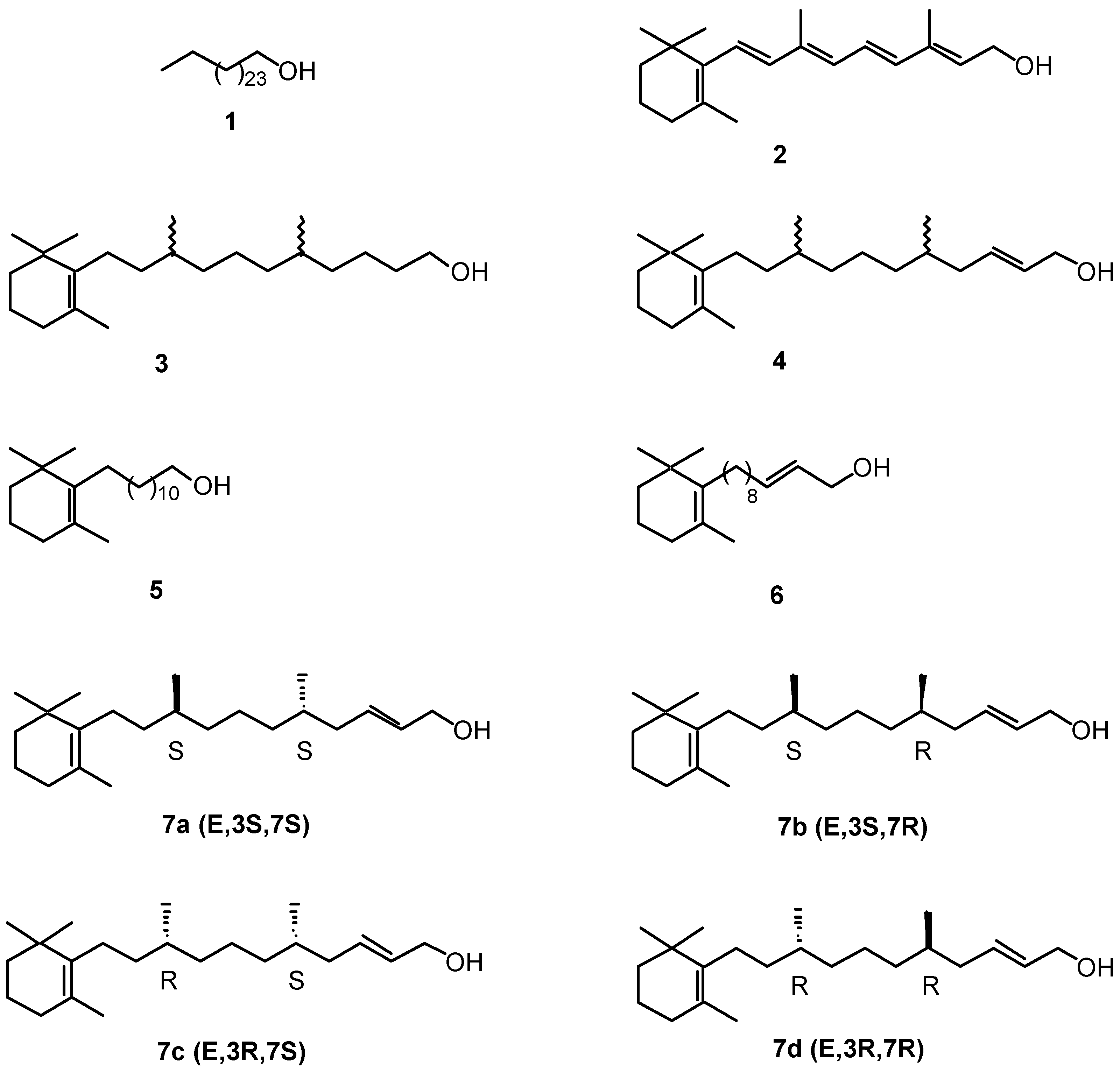
- 3. Long-chain fatty alcohols
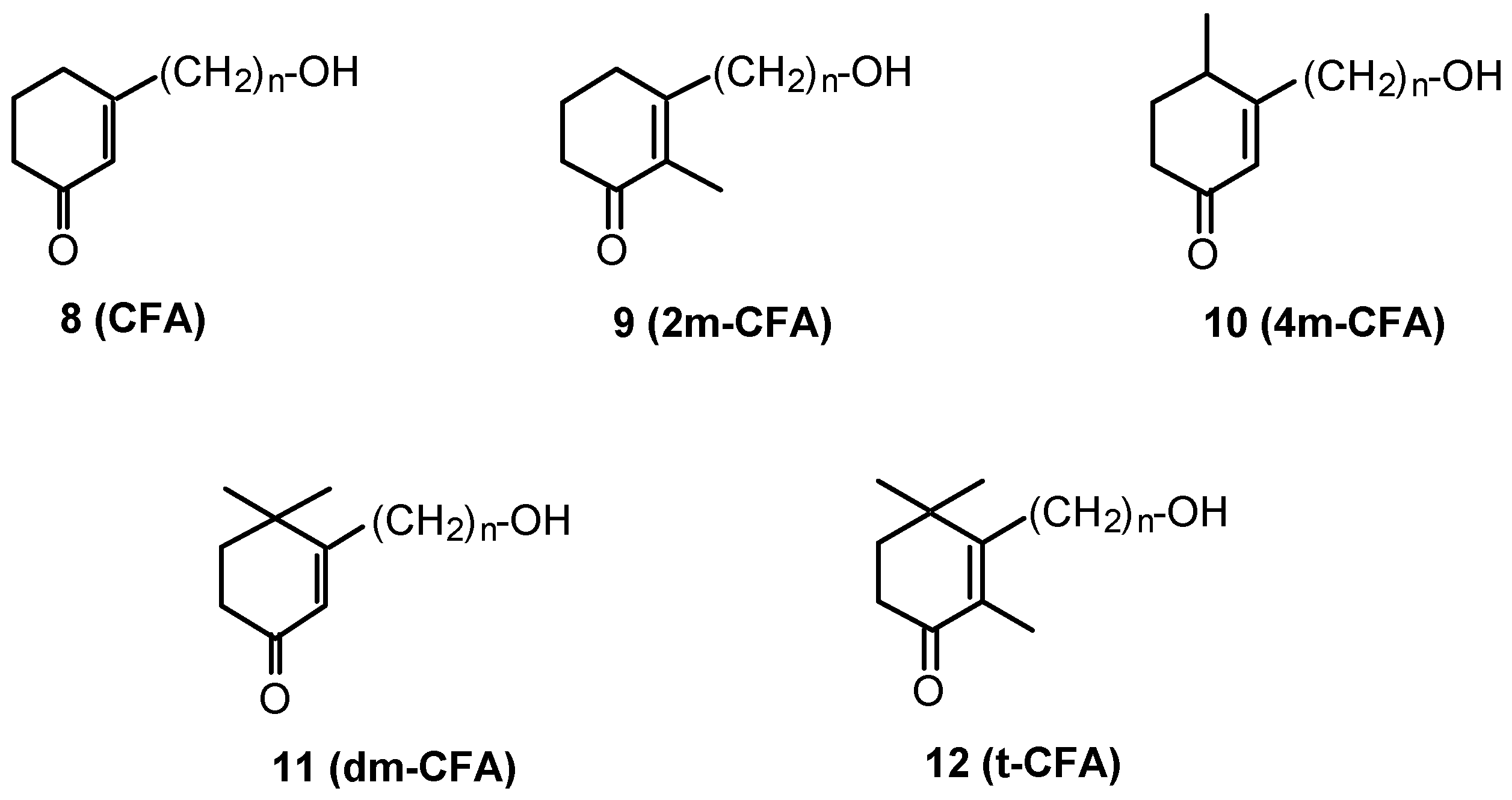
- 4. Cyclohexenonic long-chain fatty alcohols
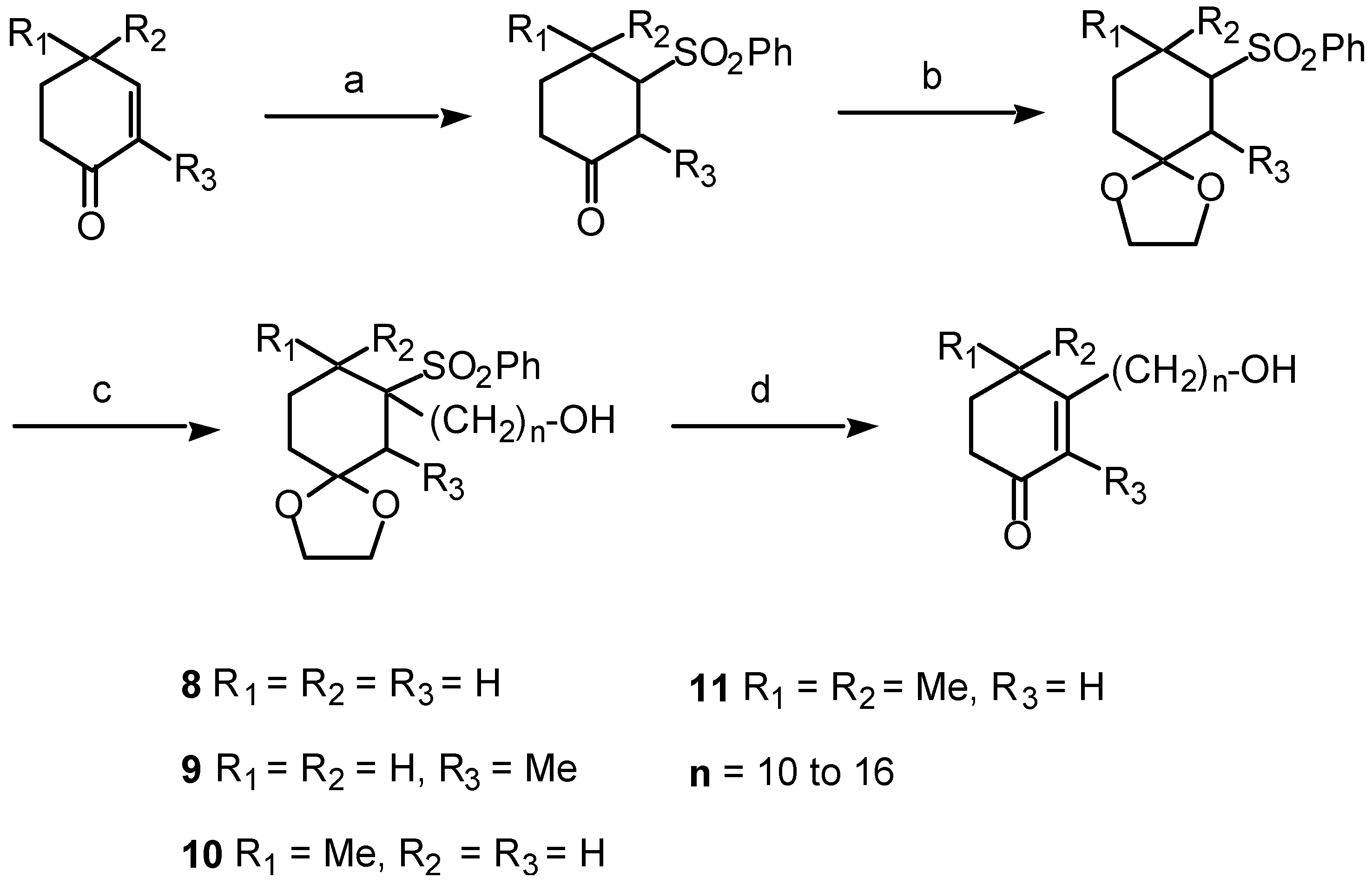
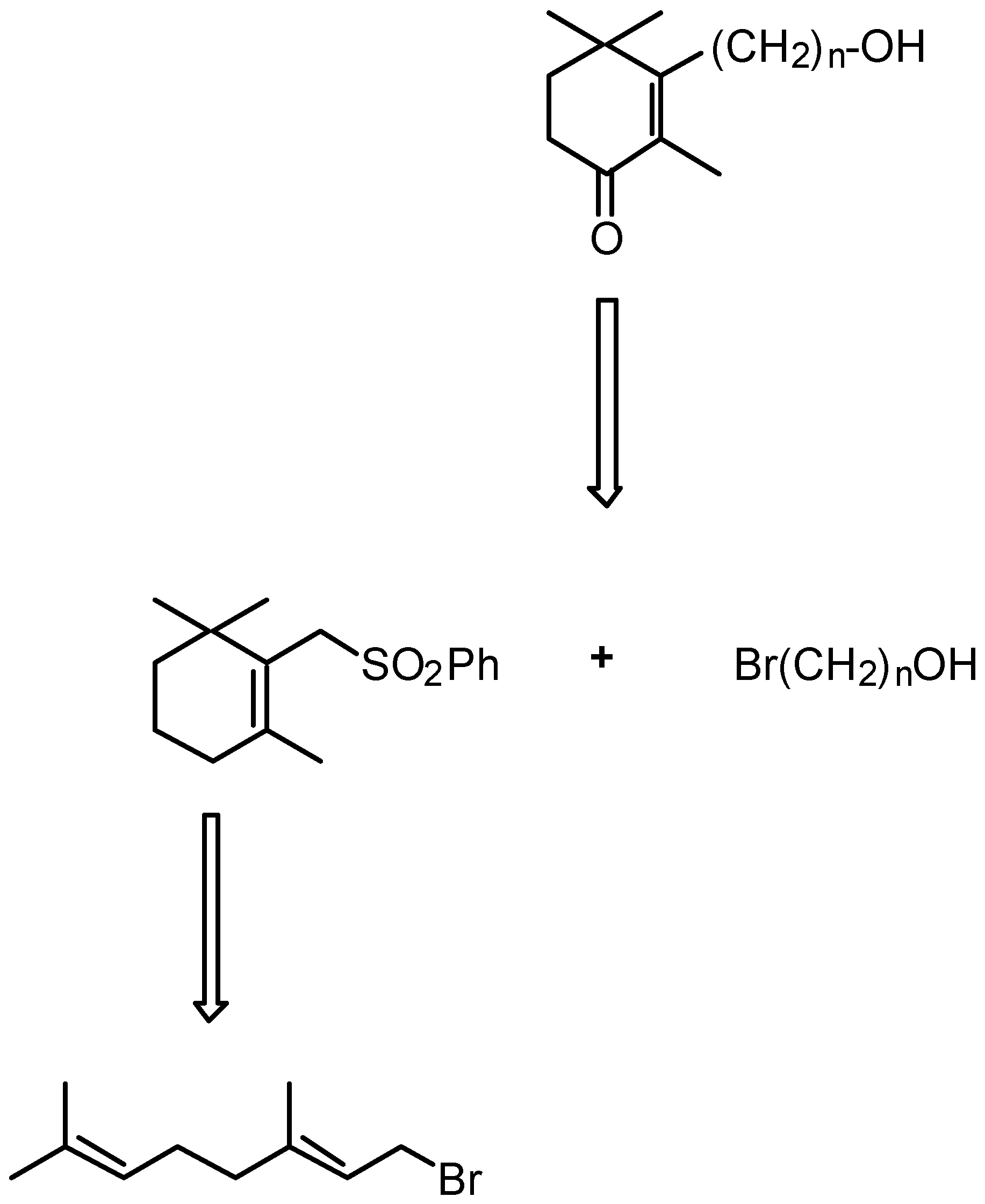
- 4.1. Synthesis of cyclohexenonic long-chain fatty alcohols
- 4.2. Biological activity of cyclohexenonic long-chain fatty alcohols
- 5. Conclusions
- 6. Experimental section: synthesis of t-CFAs
- 6.1. Materials and Methods
- 6.2. Synthesis of 1-(12-hydroxydodecyl-1-phenylsulfonyl)-2,6,6-trimethyl-1-cyclohexene
- 6.3. Synthesis of 1-(12-hydroxydodecyl)-2,6,6-trimethyl-1-cyclohexene
- 6.4. Synthesis of 1-(12-acetoxydodecyl)-2,6,6-trimethyl-1-cyclohexene
- 6.5. Synthesis of 3-(12-acetoxydodecyl)-2,4,4-trimethyl-2-cyclohexen-1-one
- 6.6. Synthesis of 3-(12-hydroxydodecyl)-2,4,4-trimethyl-2-cyclohexen-1-one
1. Introduction
2. Neurotrophic compounds as therapeutic agents for neurodegenerative diseases
2.1. Natural neurotrophic factors
2.2. Non-peptide neurotrophic factor analogues
3. Long-chain fatty alcohols
4. Cyclohexenonic long-chain fatty alcohols
4.1. Synthesis of cyclohexenonic long-chain fatty alcohols
4.2. Biological activity of cyclohexenonic long-chain fatty alcohols
5. Conclusions
6. Experimental section: synthesis of t-CFAs
6.1. Materials and Methods
6.2. Synthesis of 1-(12-hydroxydodecyl-1-phenylsulfonyl)-2,6,6-trimethyl-1-cyclohexene
6.3. Synthesis of 1-(12-hydroxydodecyl)-2,6,6-trimethyl-1-cyclohexene
6.4. Synthesis of 1-(12-acetoxydodecyl)-2,6,6-trimethyl-1-cyclohexene
6.5. Synthesis of 3-(12-acetoxydodecyl)-2,4,4-trimethyl-2-cyclohexen-1-one
6.6. Synthesis of 3-(12-hydroxydodecyl)-2,4,4-trimethyl-2-cyclohexen-1-one
Acknowledgements:
References
- Levi-Montalcini, R. Science 1987, 237, 1154–1162. [PubMed]
- Lindsay, R.M.; Wiegand, S.J.; Altar, C.A.; DiStefano, P.S. Trends Neurosci. 1994, 17, 182–190. [PubMed]
- Miller, R.G.; Bryan, W.W.; Dietz, M.A.; Munsat, T.L.; Petajan, J.H.; Smith, S.A.; Goodpasture, J.C. Neurology 1996, 47, 1329–1331. [PubMed]
- Miller, R.G.; Petajan, J.H.; Bryan, W.W.; Armon, C.; Barohn, R.J.; Goodpasture, J.C.; Hoagland, R.J.; Parry, G.J.; Ross, M.A.; Stromatt, S.C. Annu. Neurol. 1996, 39, 256–260.
- Seiger, A.; Nordberg, A.; von Holst, H.; Backman, L.; Ebendal, T.; Alafuzoff, I.; Amberla, K.; Hartvig, P.; Herlitz, A.; Lilja, A. Behav. Brain Res. 1993, 57, 255–261.
- Eriksdotter, J.M.; Nordberg, A.; Amberla, K.; Backman, L.; Ebendal, T.; Meyerson, B.; Olson, L.; Seiger, A.; Shigeta, M.; Theodorsson, E.; Viitanen, M.; Winblad, B.; Wahlund, L.O. Dement. Geriatr. Cogn. Disord. 1998, 9, 246–257.
- Maysinger, D.; Piccardo, P.; Liberini, P.; Jalsenjak, I.; Cuello, C. Neurochem. Int. 1994, 24, 495–503.
- Aebischer, P.; Schluep, M.; Deglon, N.; Joseph, J.M.; Hirt, L.; Heyd, B.; Goddard, M.; Hammang, J.P.; Zurn, A.D.; Kato, A.C.; Regli, F.; Baetge, E.E. Nat. Med. 1996, 2, 696–699. [CrossRef] [PubMed]
- Bilang-bleuel, A.; Revah, F.; Colin, P.; Locquet, I.; Robert, J.J.; Mallet, J.; Horellou, P. Proc. Natl. Acad. Sci. USA 1997, 94, 8818–8823. [CrossRef]
- Haase, G.; Kennel, P.; Pettmann, B.; Vigne, E.; Akli, S.; Revah, F.; Schmalbruch, H.; Kahn, A. Nat. Med. 1997, 3, 429–436.
- Friden, P.M.; Walus, L.R.; Watson, P.; Doctrow, S.R.; Kozarich, J.W.; Backman, C.; Bergman, H.; Hoffer, B.; Bloom, F.; Granholm, A.C. Science 1993, 259, 373–377. [PubMed]
- LeSauteur, L.; Wei, L.; Gibbs, B.F.; Saragovi, H.U. J. Biol. Chem. 1995, 270, 6564–6569.
- Xie, Y.; Tisi, M.A.; Yeo, T.T.; Longo, F.M. J. Biol. Chem. 2000, 275, 29868–29874. [CrossRef] [PubMed]
- Omura, S.; Fujimoto, T.; Otoguro, K.; Matsuzaki, K.; Moriguchi, R.; Tanaka, H.; Sasaki, Y. J. Antibiot. 1991, 44, 113–116. [CrossRef]
- Toki, S.; Ando, K.; Yoshida, M.; Matsuda, Y. J. Antibiot. 1994, 47, 1175–1181. [CrossRef]
- Kakeya, H.; Takahashi, I.; Okada, G.; Isono, K.; Osada, H. J. Antibiot. 1995, 48, 733–735. [CrossRef] [PubMed]
- Fenteany, G.; Schreiber, S.L. J. Biol. Chem. 1998, 273, 8545–8548. [CrossRef]
- Mizushina, Y.; Kobayashi, S.; Kuramochi, K.; Nagata, S.; Sugawara, F.; Sakaguchi, K. Biochem. Biophys. Res. Commun. 2000, 273, 784–788. [CrossRef] [PubMed]
- Fukuyama, Y.; Otoshi, Y.; Miyoshi, K.; Nakamura, K.; Kodama, M.; Nagasawa, M.; Hasegawa, T.; Okazaki, H.; Sugawara, M. Tetrahedron 1992, 48, 377–392. [CrossRef]
- Moriguchi, T.; Matsuura, H.; Kodera, Y.; Itakura, Y.; Katsuki, H.; Saito, H.; Nishiyama, N. Neurochem. Res. 1997, 22, 1449–1452.
- Paul, J.W.; Quach, T.T.; Duchemin, A.M.; Schrier, B.K.; DaVanzo, J.P. Dev. Brain Res. 1990, 55, 21–27.
- Paul, J.W.; DaVanzo, J.P. Brain Res. Dev. Brain Res. 1992, 67, 113–120.
- Pradines, A.; Magazin, M.; Schiltz, P.; Le Fur, G.; Caput, D.; Ferrara, P. J. Neurochem. 1995, 64, 1954–1964. [CrossRef]
- Magazin, M.; Schiltz, P.; Zachayus, J.L.; Cavrois, E.; Caput, D.; Ferrara, P. Brain Res. Mol. Brain Res. 1998, 53, 301–306. [CrossRef]
- Duong, F.H.; Warter, J.M.; Poindron, P.; Passilly, P. Br. J. Pharmacol. 1999, 128, 1385–1392.
- Gauthier, T.; Bornia, J.; Reith, Y.; Keane, P.E.; Le Fur, G.; Soubrie, P. Fund. Clin. Parmacol. 1993, 7, 359.
- Labie, C.; Lafon, C.; Marmouget, C.; Saubusse, P.; Fournier, J.; Keane, P.E.; Le Fur, G.; Soubrie, P. Br. J. Pharmacol. 1999, 127, 139–144.
- Fournier, J.; Steinberg, R.; Gauthier, T.; Keane, P.E.; Guzzi, U.; Coude, F.X.; Bougault, I.; Maffrand, J.P.; Soubrie, P.; Le Fur, G. Neuroscience 1993, 55, 629–641. [PubMed]
- Terranova, J.P.; Kan, J.P.; Storme, J.J.; Perreaut, P.; Le Fur, G.; Soubrie, P. Neurosci. Lett. 1996, 213, 79–82.
- Duong, F.H.; Fournier, J.; Keane, P.E.; Guenet, J.L.; Soubrie, P.; Warter, J.M.; Borg, J.; Poindron, P. Br. J. Pharmacol. 1998, 124, 811–817.
- Iwasaki, I.; Shiojima, T.; Kinoshita, M.; Ikeda, K. J. Neurol. Sci. 1998, 160, 92–96. [CrossRef]
- Furukawa, Y.; Tomioka, N.; Sato, W.; Satoyoshi, E.; Hayashi, K.; Furukawa, S. FEBS Lett. 1989, 247, 463–467. [PubMed]
- Hanaoka, Y.; Ohi, T.; Furukawa, S.; Furukawa, Y.; Hayashi, K.; Matsukura, S. J. Neurol. Sci. 1994, 122, 28–32. [CrossRef]
- Kaechi, K.; Ikegami, R.; Nakamura, N.; Nakajima, M.; Furukawa, Y.; Furukawa, S. J. Pharmacol. Exp. Ther. 1995, 272, 1300–1304. [PubMed]
- Saita, K.; Ohi, T.; Hanaoka, Y.; Furukawa, S.; Furukawa, Y.; Hayashi, K.; Matsukura, S. Neurotoxicology 1995, 16, 403–412. [PubMed]
- Saita, K.; Ohi, T.; Hanaoka, Y.; Furukawa, S.; Furukawa, Y.; Hayashi, K.; Matsukura, S. J. Pharmacol. Exp. Ther. 1996, 276, 231–237.
- Kimura, N.; Nishizaki, K.; Orita, Y.; Masuda, Y. Acta Otolaryngol. Suppl. 1999, 540, 12–15.
- Coyle, J.T.; Price, D.L.; Delong, M.R. Science 1983, 219, 1184–1190. [PubMed]
- Hefti, F. J. Neurosci. 1986, 6, 2155–2162.
- Olson, L.; Nordberg, A.; von Holst, H.; Backman, L.; Ebendal, T.; Alafuzoff, I.; Amberla, K.; Hartvig, P.; Herlitz, A.; Lilja, A.; Lundqvist, H.; Langstrom, B.; Meyerson, B.; Persson, A.; Viitanen, M.; Winblad, B.; Seiger, A. J. Neural Transm. Park. Dis. Dement. Sec. 1992, 4, 79–95. [CrossRef]
- Takeuchi, R.; Murase, K.; Furukawa, Y.; Furukawa, S.; Hayashi, K. FEBS Lett. 1990, 261, 63–66. [PubMed]
- Shinoda, I.; Furukawa, Y.; Furukawa, S. Biochem. Pharmacol. 1990, 39, 1813–1816.
- Rego, A.C.; Santos, M.S.; Oliveira, C.R. Free Radic. Biol. Med. 1999, 26, 1405–1417.
- Fuji, K.; Hiramatsu, M.; Kameyama, T.; Nabeshima, T. Eur. J. Pharmacol. 1993, 236, 411–417.
- Haag, P.; Schneider, T.; Schabitz, W.R.; Hacke, W. J. Neurol. Sci. 2000, 175, 52–56. [CrossRef]
- Yamada, K.; Tanaka, T.; Han, D.; Senzaki, K.; Kameyama, T.; Nabeshima, T. Eur. J. Neurosci. 1999, 11, 83–90.
- Yamada, K.; Tanaka, T.; Senzaki, K.; Kameyama, T.; Nabeshima, T. Eur. J. Pharmacol. 1998, 349, 15–22.
- Gutzmann, H.; Hadler, D. J. Neural Transm. Suppl. 1998, 54, 301–310.
- Rother, M.; Erkinjuntti, T.; Roessner, M.; Kittner, B.; Marcusson, J.; Karlsson, I. Dement. Geriatr. Cogn. Disord. 1998, 9, 36–43. [CrossRef]
- Mielke, R.; Moller, H.J.; Erkinjuntti, T.; Rosenkranz, B.; Rother, M.; Kittner, B. Alzheimer Dis. Assoc. Disord. 1998, 12, 29–35.
- Parkinson, F.E.; Paterson, A.R.P.; Young, J.D.; Cass, C.E. Biochem. Pharmacol. 1993, 46, 891–896.
- Mordente, A.; Martorana, G.E.; Minotti, G.; Giardina, B. Chem. Res. Toxicol. 1998, 11, 54–63. [CrossRef] [PubMed]
- Banati, R.B.; Schubert, P.; Rothe, G.; Gehrmann, J.; Rudulphi, K.; Valet, G.; Kreutzberg, G.W. J. Cereb. Blood Flow Metab. 1994, 14, 145–149. [CrossRef] [PubMed]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Tetrahedron Lett. 1991, 32, 4561–45-64.
- Yamaguchi, K.; Tsuji, T.; Wakuri, S.; Yazawa, K.; Kondo, K.; Higemori, H.; Kobayashi, J. Biosci. Biotech. Biochem. 1993, 57, 195–199. [CrossRef] [PubMed]
- Doi, Y.; Shigemori, H.; Ishibashi, M.; Mizobe, F.; Kawashima, A.; Nakaike, S.; Kobayashi, J. Chem. Pharm. Bull. 1993, 41, 2190–2191. [CrossRef]
- Yamaguchi, K.; Sasano, A.; Urakami, T.; Tsuji, T.; Kondo, K. Biosci. Biotech. Biochem. 1993, 57, 1231–1233. [CrossRef] [PubMed]
- Kawagishi, H.; Shimada, A.; Hosokawa, S.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S. Tetrahedron Lett. 1996, 37, 7399–7402.
- Kawagishi, H.; Ishiyama, D.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S.; Li, J. Phytochemistry 1997, 45, 1203–1205. [PubMed]
- Kita, T.; Takaya, Y.; Oshima, Y. Tetrahedron 1998, 54, 11877–11886.
- Martin, P. Science 1997, 276, 75–81. [PubMed]
- Matsuda, H.; Koyama, H.; Sato, H.; Sawada, J.; Itakura, A.; Tanaka, A.; Matsumoto, M.; Konno, K.; Ushio, H.; Matsuda, K. J. Exp. Med. 1998, 187, 297–306. [CrossRef]
- Borg, J.; Toazara, J.; Hietter, H.; Henry, M.; Schmitt, G.; Luu, B. FEBS Lett. 1987, 213, 406–410. [PubMed]
- Borg, J.; Kesslak, P.J.; Cotman, C.W. Brain Res 1990, 518, 295–298. [PubMed]
- Borg, J. J. Neurosci. Res. 1991, 29, 62–67. [CrossRef] [PubMed]
- Dawson, M.I. Curr. Pharm. Des. 2000, 6, 311–325.
- Borg, J. International Patent WO91/05754, 1991. PCT/FR90/00742 (15/10/90).
- Luu, B.; Borg, J.; Beck, A.; Laabich, A.; Crestani, F.; Neveux, O. International Patent WO94/11343, 1994. PCT/FR93/01059 (28/10/93).
- Keyling-Bilger, F.; Schmitt, G.; Beck, A.; Luu, B. Tetrahedron 1996, 52, 14891–14904.
- Girlanda-Junges, C.; Keyling-Bilger, F.; Schmitt, G.; Luu, B. Tetrahedron 1998, 54, 7735–7748.
- Luu, B.; Schmitt, G.; Keyling, F.; Girlanda, C.; Yamada, M.; Suma, Y. International Patent WO99/08987, 1999. PCT/JP98/03560 (11/08/98).
- Luu, B.; Schmitt, G.; Keyling, F.; Girlanda-Junges, C.; Chabert, P.; Loeffler, J.P.; Lutz-Bucher, B.; Gonzalez de Aguilar, J.L.; Yamada, M.; Suma, Y. International Patent WO00/47199, 2000. PCT/JP00/00742 (10/02/00).
- Krishna, H.J.V.; Joshi, B.N. J. Org. Chem. 1957, 22, 224–225. [CrossRef]
- Torii, S.; Uneyama, K.; Isihara, M. Chem Lett. 1975, 479–782.
- Trost, B.M.; Arndt, H.C.; Strege, P.E.; Verhoeven, T.H. Tetrahedron Lett. 1976, 39, 3477–3478. [CrossRef]
- Miller, R.A.; Li, W.; Humphrey, G.R. Tetrahedron Lett. 1996, 37, 3429–3432.
- Barua, N.C.; Schmidt, R.R. Synthesis 1986, 891–893.
- Borg, J.; Spitz, B.; Hamel, G.; Mark, J. Dev. Brain Res. 1985, 18, 37–49. [CrossRef]
- Kakeya, H.; Onozawa, C.; Sato, M.; Arai, K.; Osada, H. J. Med. Chem. 1997, 40, 391–394. [CrossRef] [PubMed]
- Furukawa, Y.; Furukawa, S.; Ikeda, F.; Satoyoshi, E.; Hayashi, K. FEBS Lett. 1986, 208, 258–262. [PubMed]
- Ripps, M.E.; Huntley, G.W.; Hof, P.R.; Morrison, J.H.; Gordon, J.W. Proc. Natl. Acad. Sci. USA 1995, 92, 689–693. [CrossRef]
- Gonzalez de Aguilar, J.L.; Gordon, J.W.; Rene, F.; De Tapia, M.; Lutz-Bucher, B.; Gaiddon, C.; Loeffler, J.P. Neurobiol. Dis. 2000, 7, 406–415.
- Samples Availability: Available from the authors.

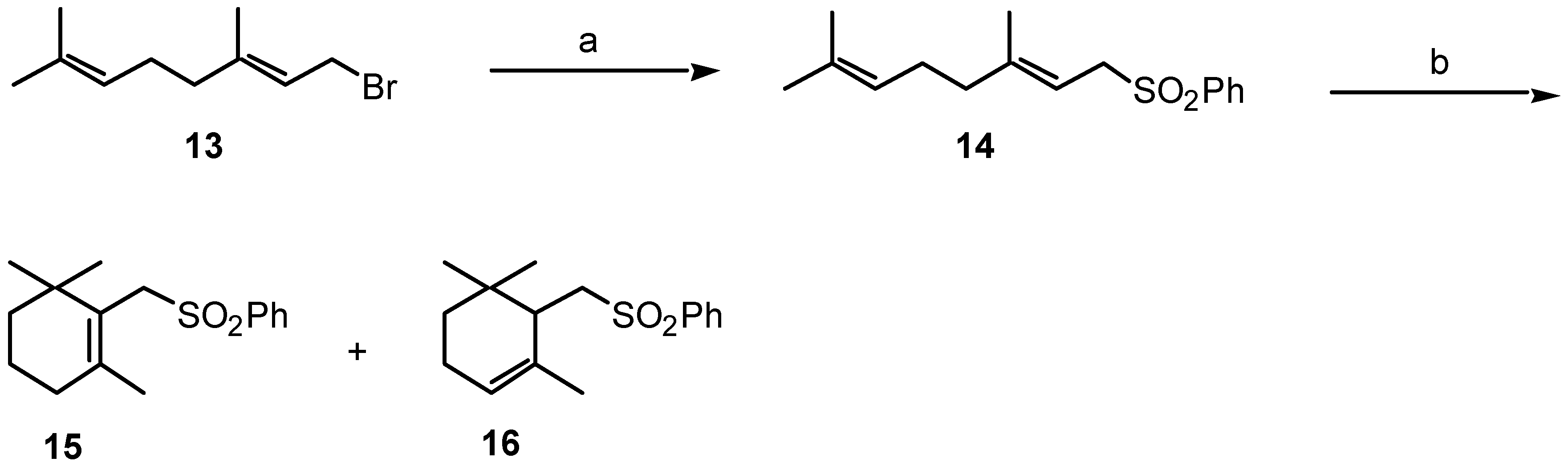

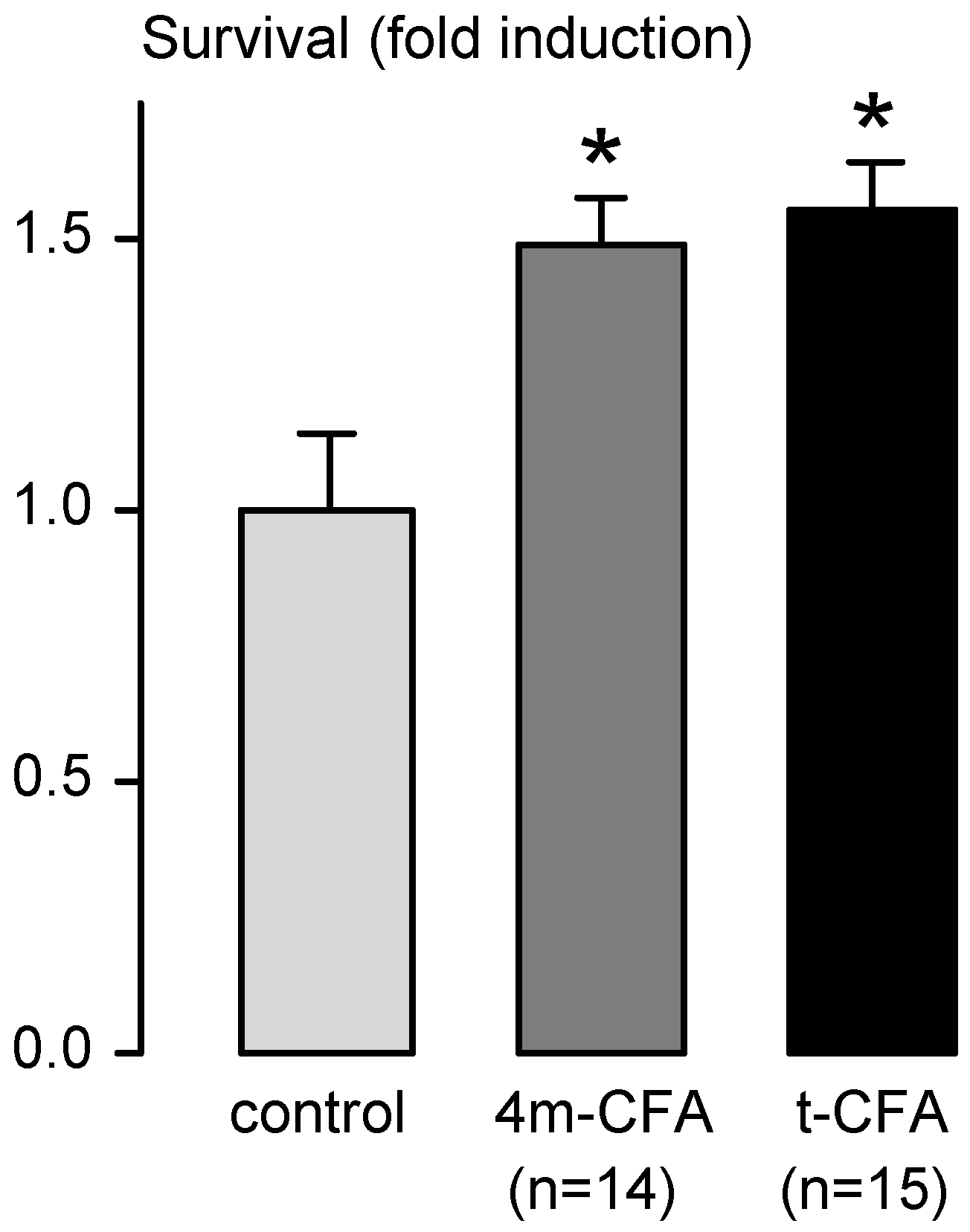
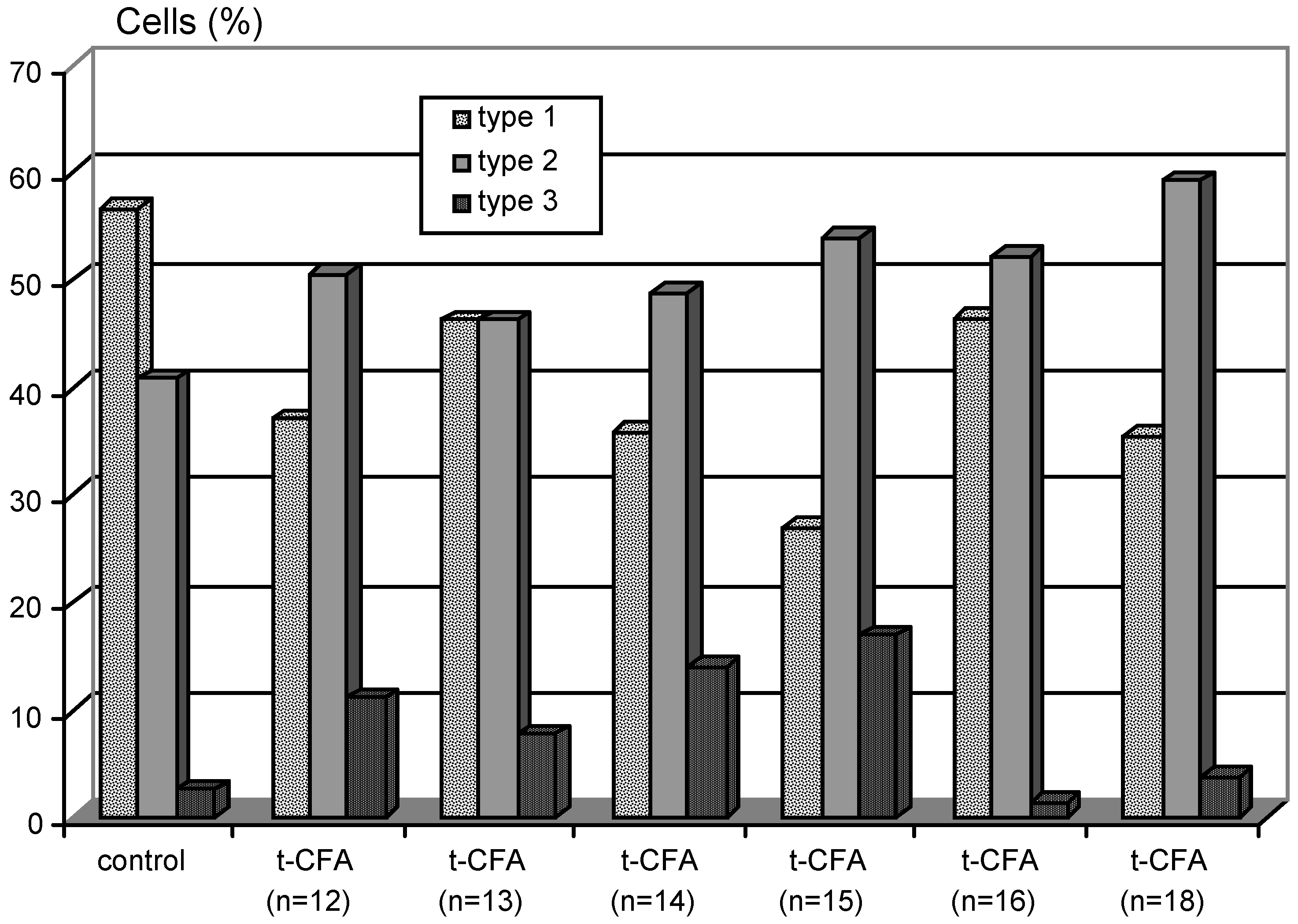
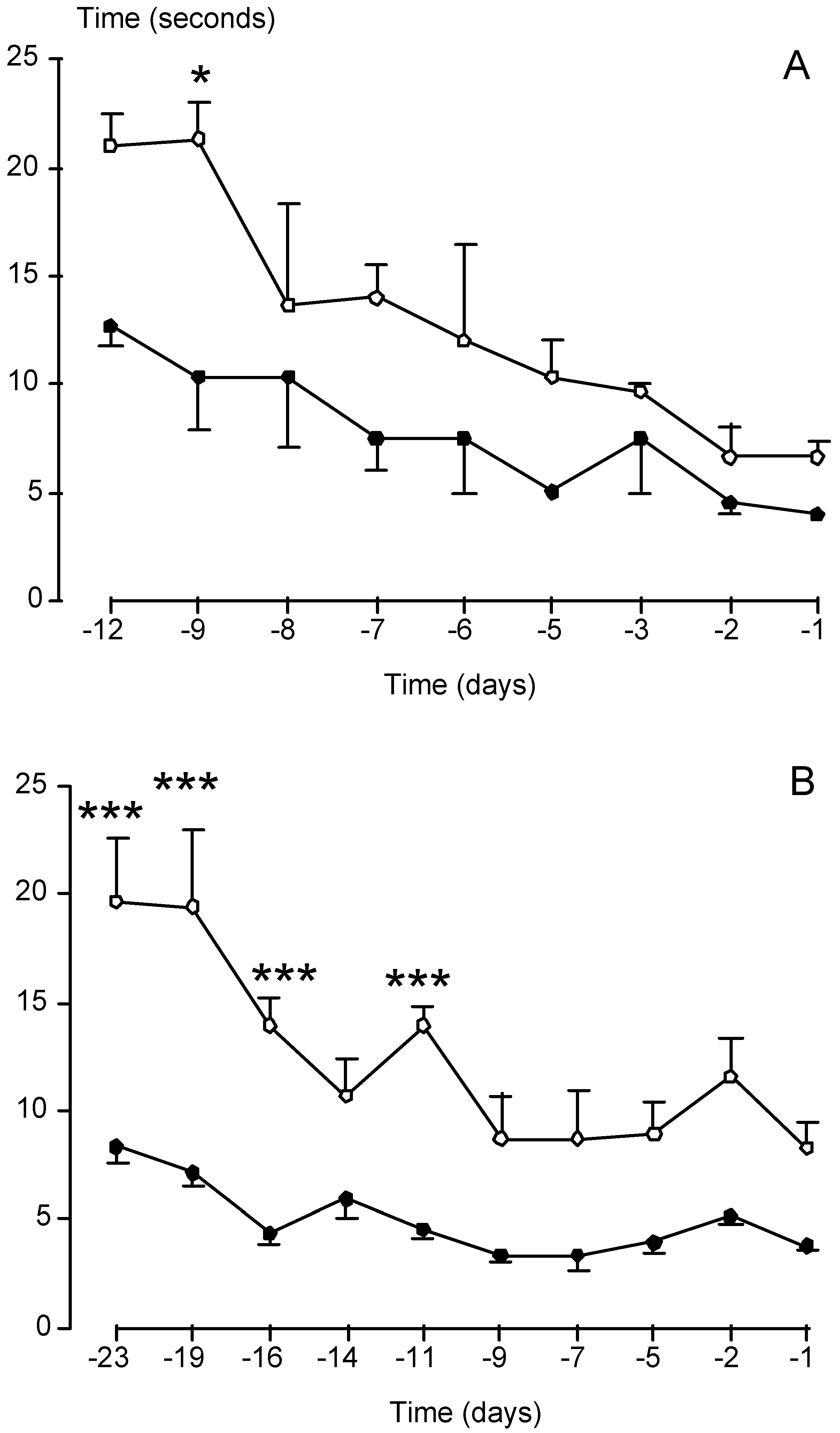
| Diseases and affected neurons | Tested Neurotrophic Factors |
|---|---|
| Alzheimer’s disease: Cholinergic neurons | NGF (Neurotrophic Growth Factor) BDNF (Brain-Derived Neurotrophic Factor) NT-4/5 (Neurotrophin-4/5) bFGF (basic Fibroblast Neurotrophic Factor) |
| Parkinson’s disease: Dopaminergic neurons | GDNF (Glial-Derived Neurotrophic Factor) BDNF NT-4/5 |
| Ischemia: Striatum, hippocampus and cortex | TGF-β1 (Transforming Growth Factor) IGF-1 (Insulin-like Growth Factor) bFGF NT-4/5 |
| Amyotrophic lateral sclerosis: Motor neurons | IGF-1 BDNF CNTF (Ciliary Neurotrophic Factor) |
 |
| Product (n) | Yield (%) | R.f* | m.p (°C) | Analysis |
|---|---|---|---|---|
| (13) | 7 | .46 | 56-57 | Microanalysis (%): C28H46O3S (462.7296) Calcd: C: 72.68; H: 10.02; O: 10.37 Found: C: 72.49; H: 10.06; O: 10.62 |
| (14) | 4 | .47 | 75-76 | Microanalysis (%): C28H46O3S (462.7296) Calcd: C: 73.06; H: 10.15; O: 10.07 Found: C: 72.83; H: 10.18; O: 9.85 MS (EI): 334.9 (M-SO2Ph, 100); 136.5 (C10H16); 122.5 (C9H15); 108.5;94.5; 80.5 |
| (15) | 6 | .46 | 58-59 | Microanalysis (%): C30H50O3S (490.7832) Calcd: C: 73.42; H: 10.27; O: 9.78 Found: C: 73.34; H: 10.34; O: 9.92 |
| (16) | 6 | .48 | 85-87 | Microanalysis (%): C31H52O3S (504.810) Calcd: C: 73.76; H: 10.38; O: 9.51 Found: C: 73.56; H: 10.37; O: 9.63 |
| (18) | 8 | .56 | 93-95 | Microanalysis (%): C33H56O3S (532.8636) Calcd: C: 74.38; H: 10.59; O: 9.01 Found: C: 73.90; H: 10.74; O: 9.15 |
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Luu, B.; De Aguilar, J.-L.G.; Girlanda-Junges, C. Cyclohexenonic Long-Chain Fatty Alcohols as Neuronal Growth Stimulators. Molecules 2000, 5, 1439-1460. https://doi.org/10.3390/51201439
Luu B, De Aguilar J-LG, Girlanda-Junges C. Cyclohexenonic Long-Chain Fatty Alcohols as Neuronal Growth Stimulators. Molecules. 2000; 5(12):1439-1460. https://doi.org/10.3390/51201439
Chicago/Turabian StyleLuu, Bang, José-Luis González De Aguilar, and Céline Girlanda-Junges. 2000. "Cyclohexenonic Long-Chain Fatty Alcohols as Neuronal Growth Stimulators" Molecules 5, no. 12: 1439-1460. https://doi.org/10.3390/51201439




