Crystal Structure of Methyl 4-Acetamido-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-ß-D-allopyranoside
Abstract
:Introduction
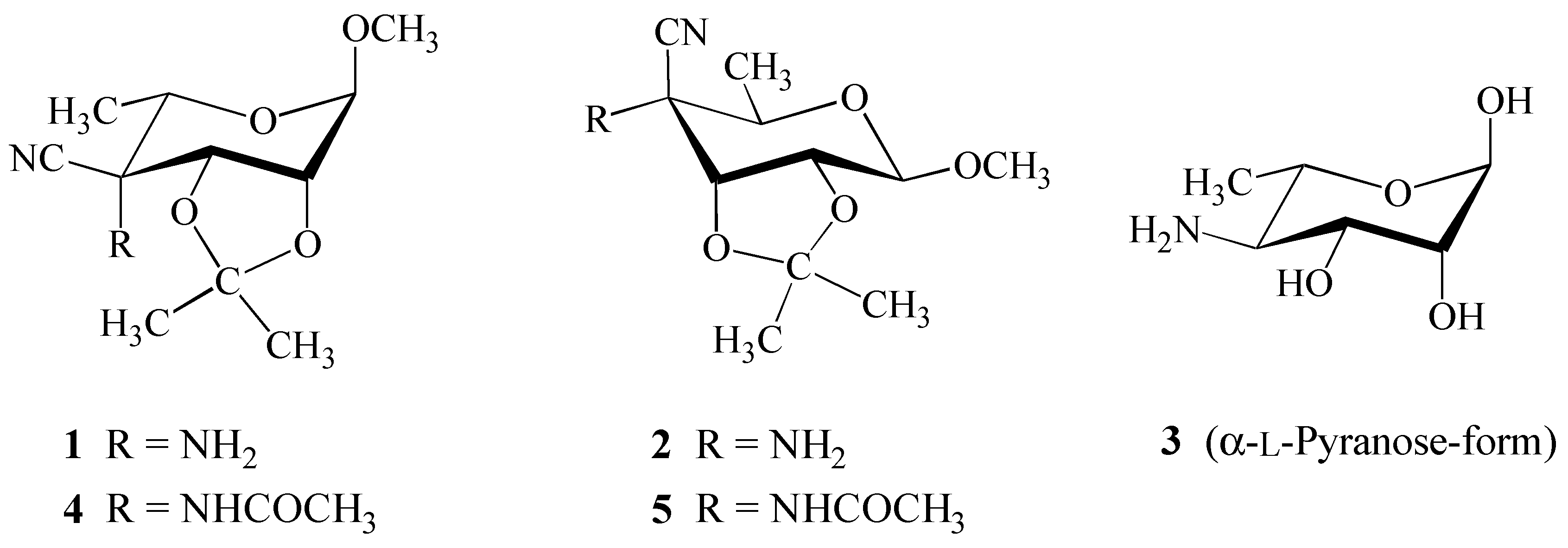
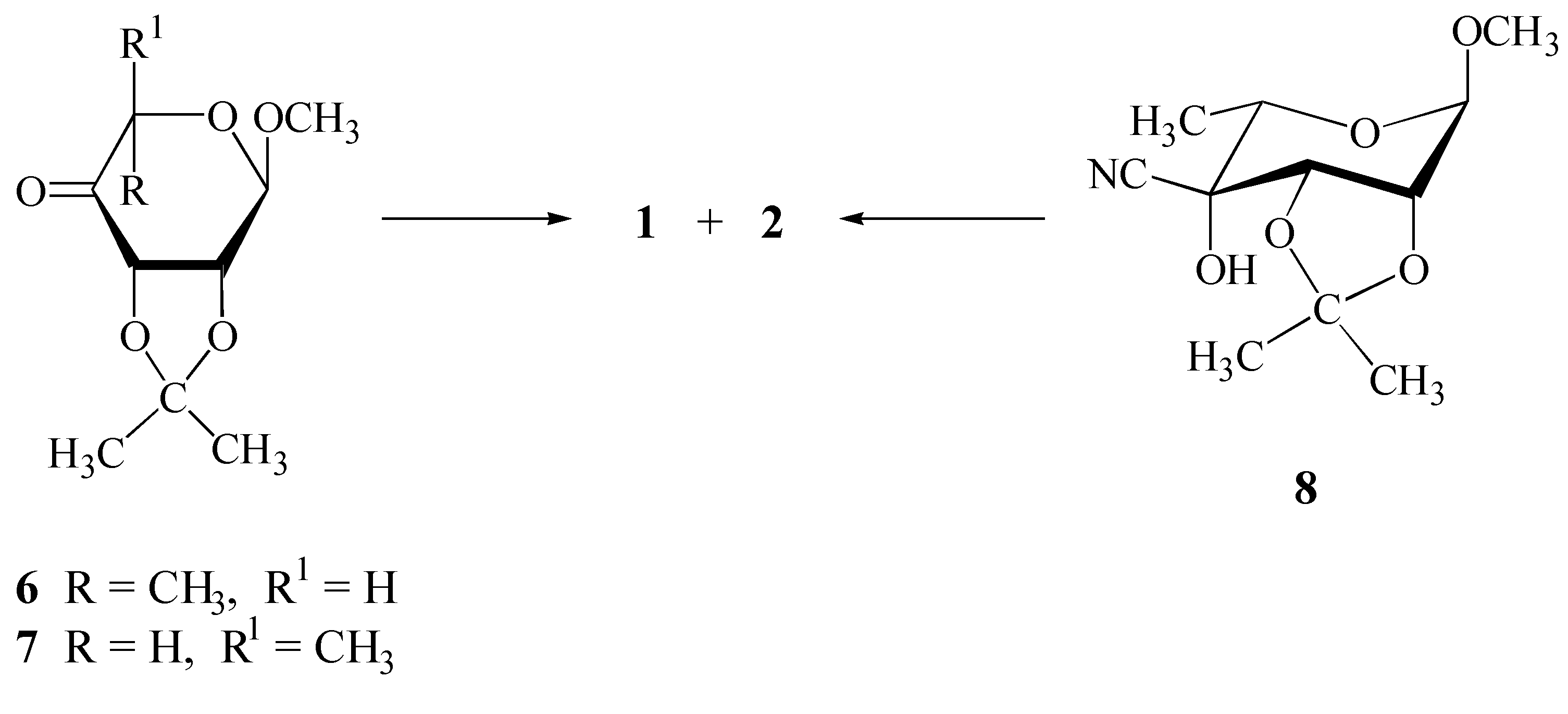
Results and Discussion
Synthesis
Structure Elucidation
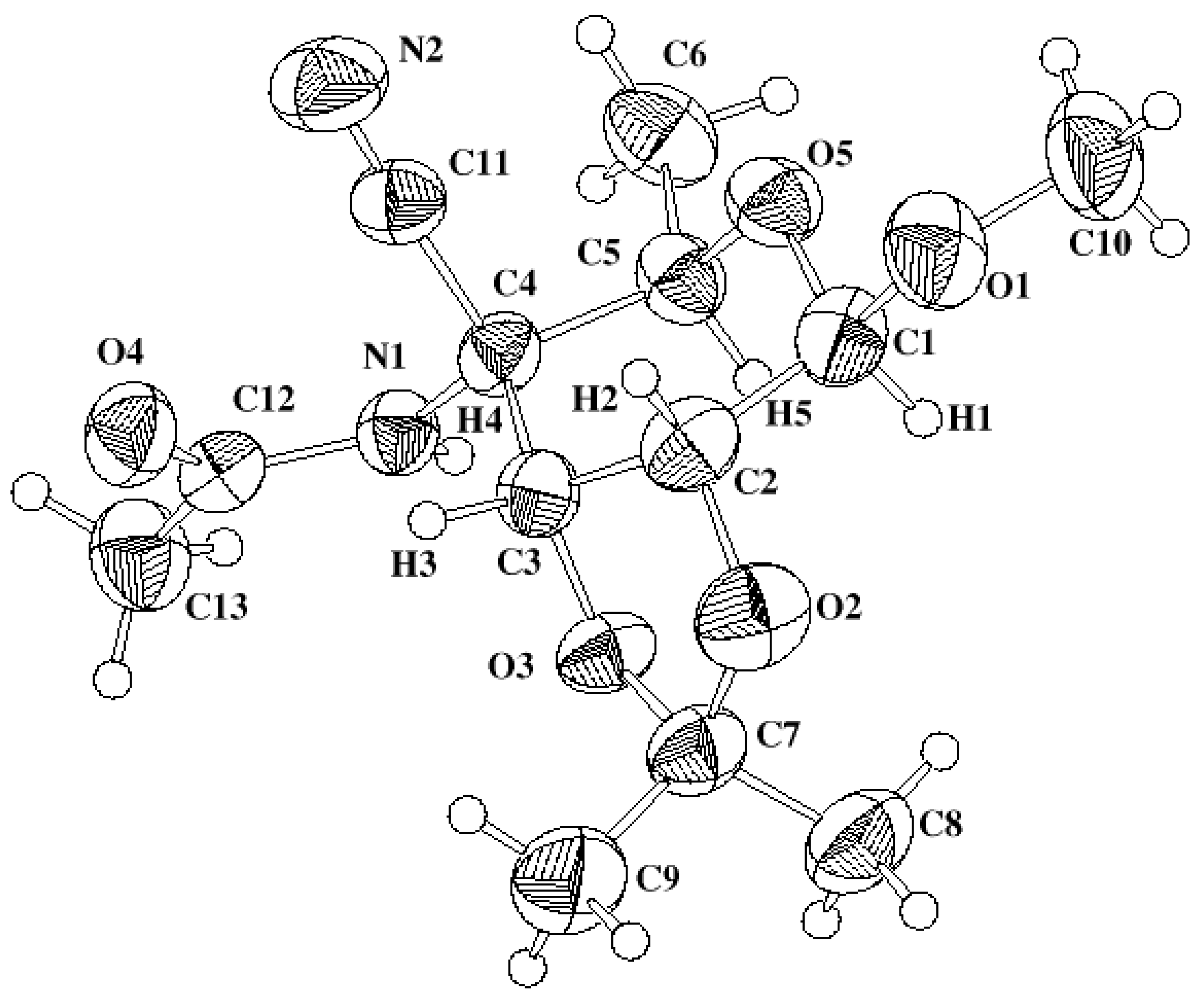
X-ray Analysis
Experimental
General
X-ray Analysis
Acknowledgements
References and Notes
- Steiner, B.; Kóǒs, M.; Langer, V.; Gyepesová, D.; Smrčok, L. 4-Amino-4-cyano-4,6-dideoxy Sugar Derivatives from Methyl 6-deoxy-2,3-O-isopropylidene-α-L-lyxo-hexopyranosid-4-ulose via Strecker-type Reaction. Carbohydr. Res. 1998, 311, 1–9. [Google Scholar] [CrossRef]
- Horton, D.; Just, E.K. Stereospecific chain-branching by C-alkylation at the ketonic and enolic positions of 1,6-anhydro-2,3-O-isopropylidene-β-D-lyxo-hexopyranos-4-ulose. Carbohydr. Res. 1971, 18, 81–94. [Google Scholar] [CrossRef]
- Stevens, C.L.; Balasubramanian, K.K.; Bryant, Ch.P.; Filippi, J.B.; Pillai, P.M. J. Org. Chem. 1973, 38, 4311–4318. [CrossRef]
- Cremer, D.; Pople, J. A. A General Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Köll, P.; Saak, W.; Pohl, S.; Steiner, B.; Koóš, M. Preparation and crystal and molecular structure of 6-O-[(2S)-2,3-epoxypropyl]-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose. Pyranoid ring conformation in 1,2:3,4-di-O-isopropylidene-galactopyranose and related systems. Carbohydr. Res. 1994, 265, 237–248. [Google Scholar] [CrossRef]
- Boeyens, J.C.A. The conformation of six-membered rings. J. Cryst. Mol. Struct. 1978, 8, 317–320. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spectroscopic Methods in Organic Chemistry; Enders, D., Noyori, R., Trost, B.M., Eds.; Georg Thieme Verlag Stuttgart: New York, 1997; p. 108. [Google Scholar]
- Bruker AXS Inc. SHELXTL Version 5.10, Madison, Wisconsin: USA, 1997.
- Zsolnai, L.; Huttner, G. Program ZORTEP; University of Heidelberg: Germany, 1994. [Google Scholar]
- Samples Availability: Available from the authors.
| Empirical formula | C13H20N2O5 |
| Formula weight | 284.31 |
| Temperature, T (K) | 296(2) |
| Wavelength, λ (Å) | 0.71073 |
| Crystal system | Hexagonal |
| Space group | P62 |
| Unit cell dimensions (Å) | a = 15.6124(6) α = β = 90° |
| b = 15.6124(6) | |
| c = 10.6318(6) γ = 120° | |
| Unit-cell volume, V (Å3) | 2244.3(2) |
| Formula units per unit cell, Z | 6 |
| Calculated density, Dx (g cm–3) | 1.262 |
| Absorption coefficient, μ (mm–1) | 0.097 |
| F(000) | 912 |
| Crystal size (mm) | 0.56 (max) 0.04 (min) |
| Diffractometer | Siemens SMART CCD |
| Theta range for data collection (°) | 1.51—23.29 |
| Index ranges | –17 ≤ h ≤ 17, -17 ≤ k ≤ 15, -11 ≤ l ≤ 11 |
| Reflections collected | 8776 |
| Independent reflections [I > 2σ(I)] | 2141 (Rint = 0.044) |
| Refinement method | Full-matrix least-squares on F2 |
| Data / parameters | 2141 / 206 |
| Goodness of fit (all) | 1.013 |
| Final R indices [I > 2σ(I)] | R1 = 0.0490, wR2 = 0.1232 |
| R indices (all data) | R1 = 0.0706, wR2 = 0.1443 |
| Largest diff. peak and hole | 0.133 and –0.169 (e Å–3) |
| C4–C11 | 1.495(7) | C4–N1 | 1.449(5) |
| C4–C3 | 1.540(5) | C4–C5 | 1.546(5) |
| C3–O3 | 1.414(5) | C3–C2 | 1.525(6) |
| C2–O2 | 1.422(5) | C2–C1 | 1.512(6) |
| C1–O1 | 1.377(5) | C1–O5 | 1.429(5) |
| O5–C5 | 1.412(5) | C5–C6 | 1.507(6) |
| O3–C7 | 1.428(5) | O2–C7 | 1.439(5) |
| C7–C8 | 1.494(7) | C7–C9 | 1.509(6) |
| O1–C10 | 1.449(6) | C11–N2 | 1.133(6) |
| N1–C12 | 1.334(5) | O4–C12 | 1.238(5) |
| C12–C13 | 1.490(6) | C11–C4–N1 | 110.9(3) |
| C11–C4–C3 | 107.2(3) | N1–C4–C3 | 111.9(3) |
| C11–C4–C5 | 107.9(3) | N1–C4–C5 | 109.5(3) |
| C3–C4–C5 | 109.3(3) | O3–C3–C2 | 102.0(3) |
| O3–C3–C4 | 108.3(3) | C2–C3–C4 | 115.7(3) |
| O2–C2–C1 | 110.8(3) | O2–C2–C3 | 102.5(3) |
| C1–C2–C3 | 114.1(3) | O1–C1–O5 | 108.0(3) |
| O1–C1–C2 | 108.4(3) | O5–C1–C2 | 111.5(3) |
| C5–O5–C1 | 111.9(3) | O5–C5–C4 | 107.5(3) |
| O5–C5–C6 | 107.9(3) | C4–C5–C6 | 114.5(4) |
| C3–O3–C7 | 106.2(3) | C7–O2–C2 | 108.7(3) |
| O2–C7–O3 | 105.8(3) | O2–C7–C8 | 110.2(4) |
| O3–C7–C8 | 108.6(4) | O2–C7–C9 | 108.5(4) |
| O3–C7–C9 | 110.4(4) | C8–C7–C9 | 113.1(4) |
| C1–O1–C10 | 113.7(4) | N2–C11–C4 | 176.5(5) |
| C12–N1–C4 | 124.7(3) | O4–C12–N1 | 120.7(4) |
| O4–C12–C13 | 121.8(4) | N1–C12–C13 | 117.4(4) |
| C1–C2 – C3–C4 | –32.8(5) |
| H1–C1–C2–H2 | 156.2(5) |
| H2–C2–C3–H3 | –35.0(7) |
| C3–C4 – C5–O5 | –57.9(4) |
| C3–C2 – O2–C7 | 20.3(5) |
| C3–C4 – C5–C6 | –177.8(4) |
| O1–C1 – C2–O2 | –84.7(4) |
| C10–O1 – C1–O5 | –64.5(5) |
| C10–O1 – C1–C2 | 174.5(4) |
| C13–C12 – N1–C4 | –178.9(4) |
| O4–C12 – N1–C4 | 3.2(7) |
| O2–C2 – C3–O3 | –35.5(5) |
| O3–C3 – C4–C11 | 170.0(3) |
| Atom | x | y | z | U(eq) |
|---|---|---|---|---|
| C4 | 2055(3) | 4584(3) | 2104(4) | 40.0(9) |
| C3 | 1000(3) | 3989(3) | 1565(4) | 41.3(9) |
| H3 | 584(3) | 4240(3) | 1904(4) | 32(9) |
| C2 | 492(3) | 2871(3) | 1736(4) | 49.9(11) |
| H2 | 119(3) | 2682(3) | 2527(4) | 61(12) |
| C1 | 1188(3) | 2462(3) | 1702(4) | 49.8(10) |
| H1 | 1345(3) | 2398(3) | 826(4) | 35(9) |
| O5 | 2080(2) | 3080(2) | 2375(2) | 50.3(8) |
| C5 | 2622(3) | 4029(3) | 1829(4) | 45.5(10) |
| H5 | 2653(3) | 3957(3) | 917(4) | 63(13) |
| O3 | 1058(2) | 4084(2) | 241(3) | 46.9(7) |
| O2 | –175(2) | 2514(2) | 702(3) | 64.1(9) |
| C7 | 159(3) | 3280(3) | –236(4) | 52.6(11) |
| C9 | –614(4) | 3583(4) | –382(6) | 77(2) |
| H9A | –391(10) | 4110(16) | –985(21) | 105(23) |
| H9B | –721(16) | 3805(21) | 414(8) | 69(15) |
| H9C | –1221(7) | 3028(7) | –669(28) | 64(13) |
| C8 | 381(4) | 2933(4) | –1438(5) | 70.7(14) |
| H8A | 671(19) | 3471(7) | –2026(10) | 71(15) |
| H8B | –220(5) | 2405(14) | –1783(14) | 74(14) |
| H8C | 835(15) | 2700(18) | –1277(6) | 89(19) |
| O1 | 727(2) | 1546(2) | 2267(3) | 67.8(9) |
| C10 | 1284(4) | 1034(4) | 2189(6) | 81(2) |
| H10A | 892(12) | 374(11) | 2512(35) | 131(25) |
| H10B | 1879(15) | 1383(17) | 2676(30) | 113(24) |
| H10C | 1451(25) | 1004(26) | 1327(7) | 120(24) |
| C6 | 3657(3) | 4519(4) | 2355(5) | 63.7(13) |
| H6A | 4039(7) | 5170(9) | 2004(21) | 69(14) |
| H6B | 3960(8) | 4133(12) | 2143(24) | 74(14) |
| H6C | 3632(3) | 4566(19) | 3253(6) | 95(19) |
| C11 | 1959(3) | 4623(3) | 3498(5) | 46.1(10) |
| N2 | 1865(3) | 4602(3) | 4557(4) | 69.1(11) |
| N1 | 2592(2) | 5571(2) | 1576(3) | 40.1(8) |
| H4 | 3041(2) | 5687(2) | 1027(3) | 63(15) |
| O4 | 1834(2) | 6207(2) | 2705(3) | 57.6(8) |
| C12 | 2440(3) | 6313(3) | 1880(4) | 44.5(9) |
| C13 | 3050(4) | 7276(3) | 1223(5) | 63.2(13) |
| H13A | 2642(9) | 7384(19) | 644(37) | 184(42) |
| H13B | 3575(24) | 7261(15) | 772(41) | 138(27) |
| H13C | 3325(32) | 7801(5) | 1830(7) | 179(35) |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Koóš, M.; Steiner, B.; Gajdoš, J.; Langer, V.; Gyepesová, D.; Smrčok, L.; Ďurík, M. Crystal Structure of Methyl 4-Acetamido-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-ß-D-allopyranoside. Molecules 2000, 5, 219-226. https://doi.org/10.3390/50300219
Koóš M, Steiner B, Gajdoš J, Langer V, Gyepesová D, Smrčok L, Ďurík M. Crystal Structure of Methyl 4-Acetamido-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-ß-D-allopyranoside. Molecules. 2000; 5(3):219-226. https://doi.org/10.3390/50300219
Chicago/Turabian StyleKoóš, Miroslav, Bohumil Steiner, Ján Gajdoš, Vratislav Langer, Dalma Gyepesová, L'ubomìr Smrčok, and Marián Ďurík. 2000. "Crystal Structure of Methyl 4-Acetamido-4-cyano-4,6-dideoxy-2,3-O-isopropylidene-ß-D-allopyranoside" Molecules 5, no. 3: 219-226. https://doi.org/10.3390/50300219




