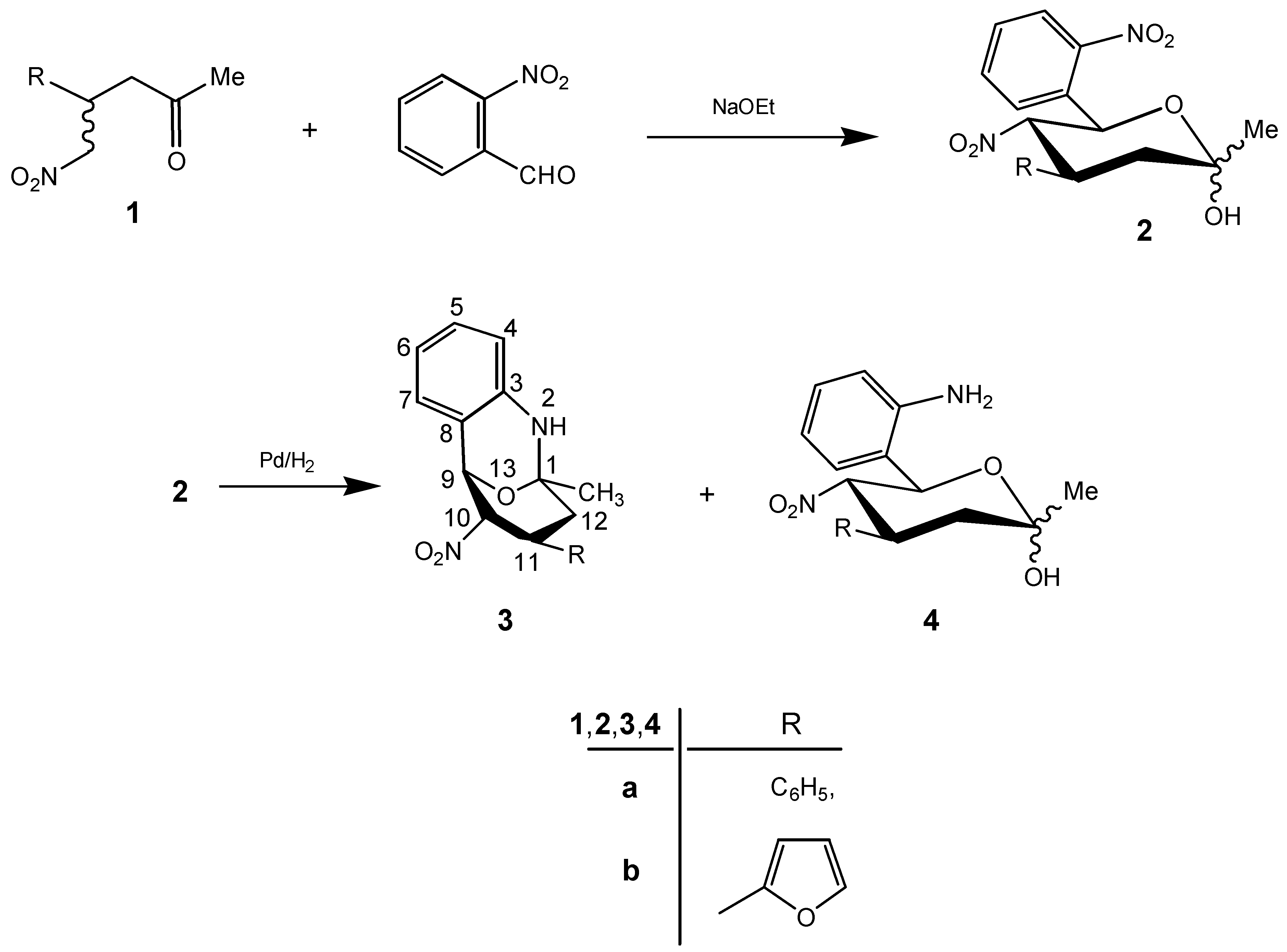
Results and Discussion
The [2
RS-(2
α, 4
β, 5
α, 6
β]-(±)-3,4,5,6-tetrahydro-2-methyl-5-nitro-6-(2-nitrophenyl)-4-phenyl(2-furyl)-2
H-pyran-2-ols
2a,
b were synthesized by treatment of (
RS)-(±)-5-nitro-4-phenyl(2-furyl)-2-pentanones (
1a,b) with 2-nitrobenzaldehyde in the presence of sodium ethoxide in dry ethanol at room temperature [
1]. The reduction of compounds
2 with palladium on charcoal in ethyl acetate yielded mixtures of (1
RS, 9
RS, 10
RS, 11
RS)-(±)-1-methyl-10-nitro-11-phenyl(2-furyl)-2-aza-13-oxa-tricyclo-[7.3.1.0
3,8]trideca-3,5,7-trienes
3a,
b and of [2
RS-(2
α, 4
β, 5
α, 6
β]-(±)-6-(2-aminophenyl)-3,4,5,6-tetrahydro-2-methyl-5-nitro-4-phenyl(2-furyl)-
2H-pyran-2-ols
4a,
b. By separation with column chromatography we obtained as main products the tricycles
3a and
3b as crystalline solids in 57 and 54% yields (
Scheme 1). The 6-(2-aminophenyl)-3,4,5,6-tetrahydro-2-methyl-5-nitro-4-phenyl(2-furyl)-
2H-pyran-2-ols
4a,
b could be isolated as by-products in yields of 28 and 26%, respectively.
Although all compounds are racemates only one isomer is shown in
Scheme 1. The analytical data of the isolated tricycles
3 are in agreement with these structures. On the other hand, the coupling constants in the
1H NMR spectra and an X-ray crystal structure investigation of compound
3b showed that during the reduction of the aromatic nitro group and the subsequent condensation the pyranoid ring system is forced into a boat conformation (
Figure 1,
Table 1). To our knowledge tricycles of this kind containing a pyranosidic ring system anellated to an aromatic ring in 1- and 2-position over a NH- function are not yet described in the literature.
Figure 1.
Molecular structure of 3b.
Figure 1.
Molecular structure of 3b.
Table 1.
Crystal-structure data.
Table 1.
Crystal-structure data.
| Empirical formula | C16 H16 N2 O4 |
| Formula weight | 300.31 |
| Unit cell dimensions [Å] | a = 7.5790(10) |
| | b = 18.069(2) |
| | c = 21.553(2) |
| | α= 90°. |
| | β = 90°. |
| | γ= 90°. |
| Volume [Å3 ] | 2951.6(6) |
| ρ (calculated) [g cm-3] | 1.352 |
| Z | 8 |
| Crystal system | Orthorhombic |
| Space group | Pbca |
| F (000) | 1264 |
| (Mo-Kα) [ mm-1 ] | 0.098 |
| Radation | 0.71073 |
| Crystal size [mm] | 0.94 x 0.44 x 0.05 |
| Data collecting mode | ω-scan |
| 2Θ range | 4.5/45 |
| hkl range | -1/8, -1/19, -1/23 |
| Measured refl. | 2561 |
| Unique refl. | 1917 |
| Observed refl. | 1474 |
| Completeness to Θ = 22.50° | 99.6% |
| Data / restraints / parameters | 1917 / 0 / 200 |
| R1 for observed | 0.0409 |
| R1 for all | 0.0590 |
| wR2 for all | 0.1104 |
| GoF2 | 1.046 |
| ρ (max/min) [e.Å-3] | 0.292 / -0.183 |
The corresponding 5-amino-2-methyl-6-(2-nitro-phenyl)-4-phenyl(2-furyl)tetrahydropyranols are interesting owing to the biological activity of a variety of 5-amino-tetrahydro-2-pyranols [
2,
3,
4]. Although there are many literature methods for conversion of a aliphatic nitro group into an amino function the 5-amino-tetrahydro-2-pyranol could not be obtained, neither by means of reducing agents like Zn/hydrochloric acid nor by refluxing with LiAlH
4 in tetrahydrofuran [
5].
Due to the 1,3-interaction of the bulky aryl groups the compounds
4, like the corresponding 2-methyl-5-nitro-6-(2-nitro-phenyl)-4-phenyl(2-furyl)tetrahydropyranols
2, possess a 1,4-chair conformation. The
J values for the couplings between the protons 3
ax-H, 4-H; 4-H, 5-H and 5H, 6-H are 10 - 13 Hz. Therefore, in all known cases the R, NH
2 and o-nitrophenyl groups are oriented in the preferred equatorial arrangement. Other diastereomers were not identified. According to the anomeric effect and the long-range coupling of the OH group with an axial proton of the ethylene group observed in the
1H NMR spectra of
4a and further investigated compounds [
6] the OH group in
4 should be in axial position.
Experimental
General
Melting points were obtained on a Boëtius melting point apparatus.
1H NMR and
13C NMR spectra were recorded on Bruker ARX 300 and AC 250 instruments with DMSO-d
6 as solvent. The calibration of spectra was carried out by means of solvent peaks (DMSO-d
6: δ
1H= 2.50; δ
13C= 39.7). Signal assignment was confirmed by DEPT and/or
1H,
13C COSY experiments. Mass spectra were recorded on an AMD 402/3 spectrometer (AMD Intectra GmbH). TLC was performed on silica gel foils 60 F
254 (Merck) with detection by charring with sulphuric acid. For column chromatography silica gel 60 (230-400 mm) (Merck) was used. Elemental analyses were carried out with a Leco CHNS-932 apparatus.
Table 1 provides a summary of the crystallographic data of compound
3b. A crystal of
3b was sealed onto a glass fiber and mounted on a Siemens P4 automated four circle diffractometer (Mo-K
α radiation;
λ = 0.71073 Å) with graphite monochromator and measured at
T = 293 K. The structure was solved by direct methods (Siemens SHELXTL, version 4.2 for MS-DOS, Siemens Analytical Xray Inst. Inc.) and refined by the full-matrix least-squares method of SHELXL-97. Non-H atoms were refined with anisotropic displacement parameters. All hydrogen atoms were placed into theoretical positions and were refined by using the riding model. Crystallographic data (excluding structure factors) reported in this paper for structure
3b have been deposited with the Cambridge Crystallo-graphic Data Centre as Supplementary Publication No. CCDC-139912. Copies of the data can be obtained free of charge on application to The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: int. code +(1223) 336-033; e-mail:
[email protected]).
Method
The [2RS-(2α, 4β, 5α, 6β]-(±)-3,4,5,6-tetrahydro-2-methyl-5-nitro-6-(2-nitrophenyl)-4-phenyl(2-furyl)-2H-pyran-2-ols 2a,b (3 mmol) were dissolved in ethyl acetate (20 mL). A small portion of palladium on charcoal (10% Pd) was added. The vessel was filled three times with hydrogen under stirring at normal pressure. After prolongation of the stirring for 48 h the solution was filtrated over Celite. Elution with ethyl acetate (3x10 mL), solvent evaporation under reduced pressure and column chromatographic separation (toluene/ ethyl acetate = 5 : 1, v/v) afforded compounds 3 and 4.
(1RS, 9RS, 10RS, 11RS)-(±)-1-Methyl-10-nitro-11-phenyl-2-aza-13-oxa-tricyclo[7.3.1.03,8]trideca-3,4,5- triene (3a)
Yield 0.53 g (57%).- m.p. 193-194oC (ether).- 1H NMR (250.1 MHz, DMSO-d6): δ/ppm = 7.32-7.10 (m, 7H, C6H5, H-5, H-7), 6.80 (m, 1H, H-6), 6.79 (s, 1H, NH), 6.70 (m, 1H, H-4), 5.45 (br s, 1H, H-9), 4.93 (dd, 1H, J10,11 = 10.4 Hz, J9,10 = 0.9 Hz, H-10), 3.35 (ddd, 1H, H-11), 2.05 (dd, 1H, J12,12• =14.3 Hz, J11,12 = 4.3 Hz, H-12), 1.66 (dd, 1H, J11,12• = 14.0 Hz, H-12´), 1.58 (s, 3H, CH3).- 13C NMR (62.9 MHz, DMSO-d6): δ/ppm = 140.1(C-3), 139.7 (i-C6H5), 128.8 (m-C6H5), 128.4, 127.4, 126.1 (C-5, C-7, p-C6H5), 127.6 (o-C6H5), 121.9 (C-8), 118.4 (C-6), 117.0 (C-4), 95.5 (C-10), 81.3 (C-1), 72.5 (C-9), 39.4 (C-12), 38.6 (C-11), 26.6 (CH3).- IR(nujol), /cm-1 = 3402 (NH), 1548, 1378 (NO2). - MS (70 eV, EI): m/z (%) = 310 (100, M+·). For C18H18N2O3 (310.1) calcd.: C 69.65 H 5.85 N 9.03; found C 69.43 H 5.81 N 8.87.
(1RS, 9RS, 10RS, 11RS)-(±)-11-(2-Furyl)-1-methyl-10-nitro-2-aza-13-oxa-tricyclo[7.3.1.03,8]trideca- 3,4,5-triene (3b)
Yield 0.48 g (54%).- m.p. 137-138oC (ether).- 1H NMR (250.1 MHz, DMSO-d6): δ/ppm = 7.45 (dd, 1H, J4-Fur,5-Fur = 1.8 Hz, J3-Fur,5-Fur = 0.6 Hz, H-5-Fur), 7.16 (m, 1H, H-7), 7.08 (m, 1H, H-5), 6.74 (m, 1H, H-6), 6.73 (s, 1H, NH), 6.62 (m, 1H, H-4), 6.26 (dd, 1H, J4-Fur,3-Fur = 3.4 Hz, H-4-Fur), 6.16 (dd, 1H, H-3-Fur), 5.45 (br s, 1H, H-9), 4.94 (dd, 1H, J10,11 = 8.9 Hz, J9,10 = 0.9 Hz, H-10), 3.60 (ddd, 1H, H-11), 2.23 (dd, 1H, J12,12• = 14.5 Hz, J11,12 = 4.6 Hz, H-12), 1.71 (dd, 1H, J11,12• = 12.3 Hz, H-12´), 1.55 (s, 3H, 13 CH3) ).- 13C NMR (62.9 MHz, DMSO-d6): δ/ppm = 152.8 (C-2-Fur), 142.4 (C-5-Fur), 140.4 (C-3), 128.4, 125.9 (C-5, C-7), 120.7 (C-8), 118.1 (C-6), 116.5 (C-4), 110.5 (C-4-Fur), 105.5 (C-3-Fur), 91.8 (C-10), 80.7 (C-1), 72.3(C-9), 36.3 (C-12), 26.9 (C-11), 25.9 (CH3).- IR(nujol), /cm-1 = 3409 (NH), 1548, 1367 (NO2). - MS (70 eV, EI): m/z (%) = 310 (100, M+·). For C16H16N2O4 (300.1) calcd. C 63.98 H 5.37 N 9.33; found C 63.98 H 5.21 N 9.25.
[2RS-(2α, 4β, 5α, 6β]-(±)-6-(2-Aminophenyl)-3,4,5,6-tetrahydro-2-methyl-5-nitro-4-phenyl-2H-pyran- 2-ol (4a)
Yield 0.27 g (28%).- m.p. 147-148oC (ethanol).- 1H NMR (250.1 MHz, DMSO-d6): δ/ppm = 7.30- 7.15 (m, 5H, C6H5), 7.07-6.94 (m, 2H, H-4-C6H4, H-6-C6H4), 6.71 (dd, 1H, J3-C6H4,4-C6H4 = 8.0 Hz, J3-C6H4,5-C6H4 = 0.9 Hz, H-3-C6H4), 6.50 (ddd, 1H, J5-C6H4,6-C6H4 = J5-C6H4,3-C6H4 = 7.5 Hz, H-5-C6H4), 6.42 (d, 1H, J3ax,OH = 1.5 Hz, OH), 5.50-5.30 (m, 2H, H-5, H-6), 5.05 (s, 2H, NH2), 3.85 (ddd, 1H, J3ax,4 = 13.0 Hz, J4,5 = 10.7 Hz, J3eq,4 = 4.0 Hz, H-4), 2.18 (ddd, 1H, H-3ax), 1.93 (dd, 1H, J3ax,3eq = 13.5 Hz, H-3eq), 1.44 (s, 3H, CH3).- 13C NMR (62.9 MHz, DMSO-d6): δ/ppm = 147.2 (C-2-C6H4), 139.6 (i-C6H5), 129.5, 129.0 (C-4-C6H5, C-6-C6H5), 128.8 (m-C6H5), 128.3 (p-C6H5), 127.5 (o-C6H5), 118.9 (C-1-C6H4), 116.3, 116.1 (C-3-C6H4, C-5-C6H4), 95.6 (C-2), 88.5 (C-5), 72.7 (C-6), 42.6 (C-4), 41.4 (C-3), 28.4 (CH3).- IR(KBr), /cm-1 = 3395, 3321 (NH2), 1545, 1336 (NO2). - MS (70 eV, EI): m/z (%) = 328 (88, M+·). For C18H20N2O4 (328.1) calcd. C 65.83 H 6.14 N 8.53; found C 65.60 H 5.94 N 8.41.
[2RS-(2α, 4β, 5α, 6β]-(±)-6-(2-Aminophenyl)-4-(2-furyl)-3,4,5,6-tetrahydro-2-methyl-5-nitro-2H-pyran- 2-ol (4b)
Yield 0.24 g (26%).- m.p. 153-154oC (ether).- 1H NMR (250.1 MHz, DMSO-d6): δ/ppm = 7.58 (dd, 1H, J5-Fur,4-Fur = 2.2 Hz, J5-Fur,3-Fur = 0.8 Hz, H-5-Fur), 7.05 (ddd, 1H, H-4-C6H4), 6.92 (dd, 1H, J6-C6H4,5-C6H4 = 7.6 Hz, J6-C6H4,4-C6H4 = 1.5 Hz, H-6-C6H4), 6.71 (dd, 1H, J3-C6H4,4-C6H4 = 8.0 Hz, J3-C6H4,5-C6H4 = 0.9 Hz, H-3-C6H4), 6.51 (ddd, 1H, J5-C6H4,4-C6H4 = 7.6 Hz, H-5-C6H4), 6.48 (br, 1H, OH), 6.39 (dd, 1H, J3-Fur,4-Fur = 3.4 Hz, H-4-Fur), 6.27 (dd, 1H, H-3-Fur), 5.33-5.22 (m, 2H, H-5, H-6), 5.02 (br, 2H, NH2), 4.00 (m, 1H, H-4), 2.20-2.00 (m, 2H, H-3ax, H-3eq), 1.46 (s, 3H, CH3).- 13C NMR (62.9 MHz, DMSO-d6): δ/ppm = 152.9 (C-2-Fur), 147.2 (C-2-C6H4), 142.8 (C-5-Fur), 129.6, 129.2 (C-4-C6H4, C-6-6H4), 118.6 (C-1-6H4), 116.4, 116.3 (C-3-6H4, C-5-6H4), 110.6 (C-4-Fur), 106.6 (C-3-Fur), 95.4 (C-2), 86.8 (C-5), 72.8 (C-6), 38.7 (C-3), 35.9 (C-4), 28.2 (CH3).- IR(nujol), /cm-1 = 3391, 3321 (NH2), 1546, 1367 (NO2). - MS (70 eV, EI): m/z (%) = 316 (18, M+·). For C16H16N2O5 (316.1) calcd. C 60.74 H 5.10 N 8.86; found C 60.86 H 5.03 N 8.79.