Synthesis and In Vitro Antigungal Properties of 4-Aryl-4-Narylamine-1-butenes and Related Compounds
Abstract
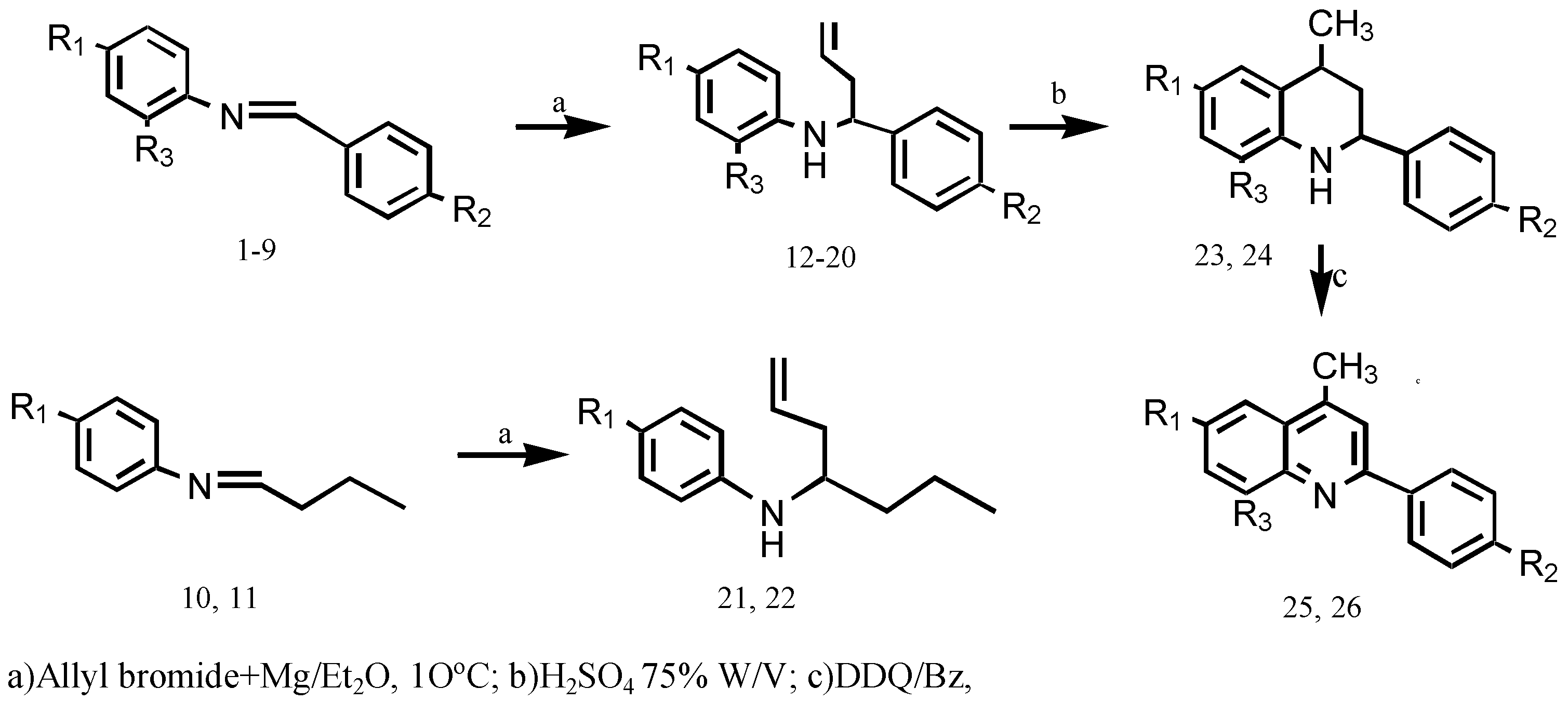
:Introduction
Experimental
Results and Discussion
References and Notes
- Zacchino, S.; Rodríguez, G.; Pezzenati, G.; Orellana, G.; Enriz, D.; González Sierra, M. J. Nat. Prod. 1997, 60, 659–662. [CrossRef] [PubMed]
- Zacchino, S.; Santecchia, C.; López, S.; Gattuso, S.; Muñoz, J.; Cruañes, A.; Vivot, E.; Salinas, A.; Ruiz, R.; Ruiz, S. Phytomedicine 1998, 5, 389–395. [PubMed]
- Rodríguez, A.; Giannini, F.; Baldoni, H.; Suvire, F.; Zacchino, S.; Sosa, C.; Enriz, R.; Csazar, P.; Czismadia, I. J. of Molecular Structure (TEOCHEM) 1999, 463, 283–303.
- Kuznetsov, V.; Andreeva, E.; Prostakov, N. Khim. Farm. Zh. 1995, 29, 61–62, (1996) Chem. Abst. 124, 48.290. This work is part of: Urbina et al., Inhibitors of the fungal wall. Synthesis of 4-aryl- 4-N-arylamine-1-butenes and related compounds with inhibitory activities on β(1-3) glucan and chitin synthases, Bioorganic & Med. Chem., in press.
 |
Share and Cite
Kouznetsov, V.; Urbina, J.; Palma, A.; López, S.; Devia, C.; Enriz, R.; Zacchino, S. Synthesis and In Vitro Antigungal Properties of 4-Aryl-4-Narylamine-1-butenes and Related Compounds. Molecules 2000, 5, 428-430. https://doi.org/10.3390/50300428
Kouznetsov V, Urbina J, Palma A, López S, Devia C, Enriz R, Zacchino S. Synthesis and In Vitro Antigungal Properties of 4-Aryl-4-Narylamine-1-butenes and Related Compounds. Molecules. 2000; 5(3):428-430. https://doi.org/10.3390/50300428
Chicago/Turabian StyleKouznetsov, V., J. Urbina, A. Palma, S. López, C. Devia, R. Enriz, and S. Zacchino. 2000. "Synthesis and In Vitro Antigungal Properties of 4-Aryl-4-Narylamine-1-butenes and Related Compounds" Molecules 5, no. 3: 428-430. https://doi.org/10.3390/50300428



