A Simple Enzymatic Preparation of 2’,3’-Di-OAcetylnucleosides Through a Lipase Catalyzed Alcoholysis
Abstract
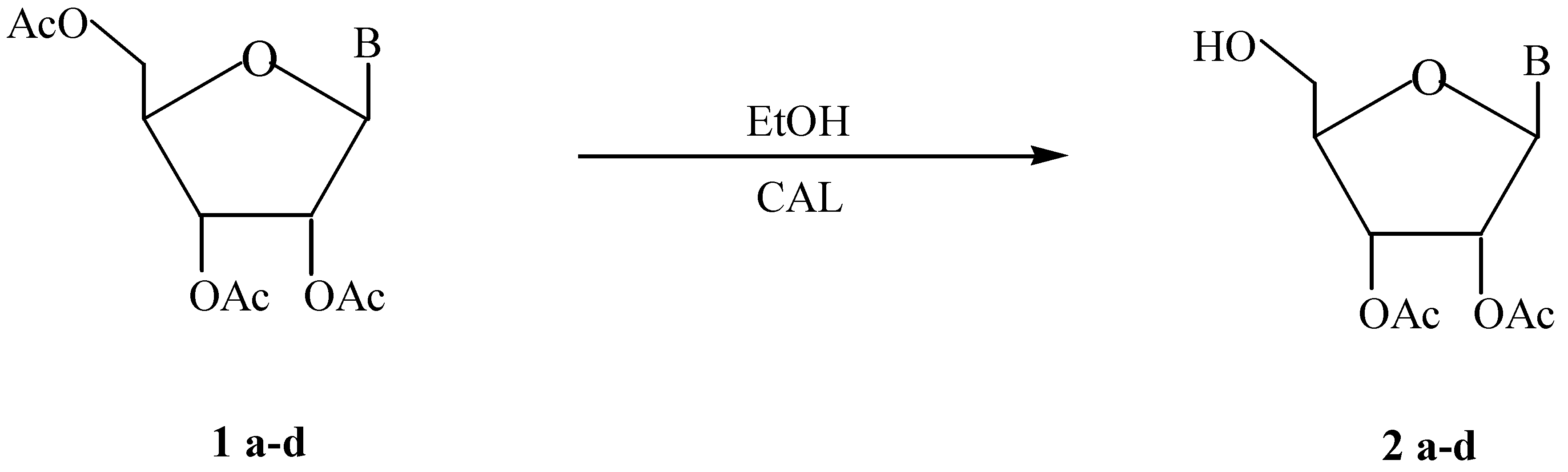
:Introduction
Experimental
Results and Discussion
References and Notes
- Périgaud, C.; Gosselin, G.; Imbach, J.L. Nucleosides Nucleotides 1992, 11, 903. [CrossRef]
- Hanrahan, J.R.; Hutchinson, D.W. J. Biotechnol. 1992, 23, 193. [PubMed]
- Wong, C.H.; Whitesides, G.M. Enzymes in synthetic organic chemistry; Elsevier Science Ltd.: Oxford, 1994; Cap. 2. [Google Scholar]
- Sachder, H.S.; Starkovsky, N.A. Tetrahedron Letters 1969, 7, 33.
- Iglesias, L.E.; Zinni, A.; Gallo, M.; Iribarren, A. Submitted for publication.
Share and Cite
Zinni, A.; Schmidt, A.; Gallo, M.; Iglesias, L.; Iribarren, A. A Simple Enzymatic Preparation of 2’,3’-Di-OAcetylnucleosides Through a Lipase Catalyzed Alcoholysis. Molecules 2000, 5, 533-534. https://doi.org/10.3390/50300533
Zinni A, Schmidt A, Gallo M, Iglesias L, Iribarren A. A Simple Enzymatic Preparation of 2’,3’-Di-OAcetylnucleosides Through a Lipase Catalyzed Alcoholysis. Molecules. 2000; 5(3):533-534. https://doi.org/10.3390/50300533
Chicago/Turabian StyleZinni, Alejandra, Alejandro Schmidt, Mariana Gallo, Luis Iglesias, and Adolfo Iribarren. 2000. "A Simple Enzymatic Preparation of 2’,3’-Di-OAcetylnucleosides Through a Lipase Catalyzed Alcoholysis" Molecules 5, no. 3: 533-534. https://doi.org/10.3390/50300533



