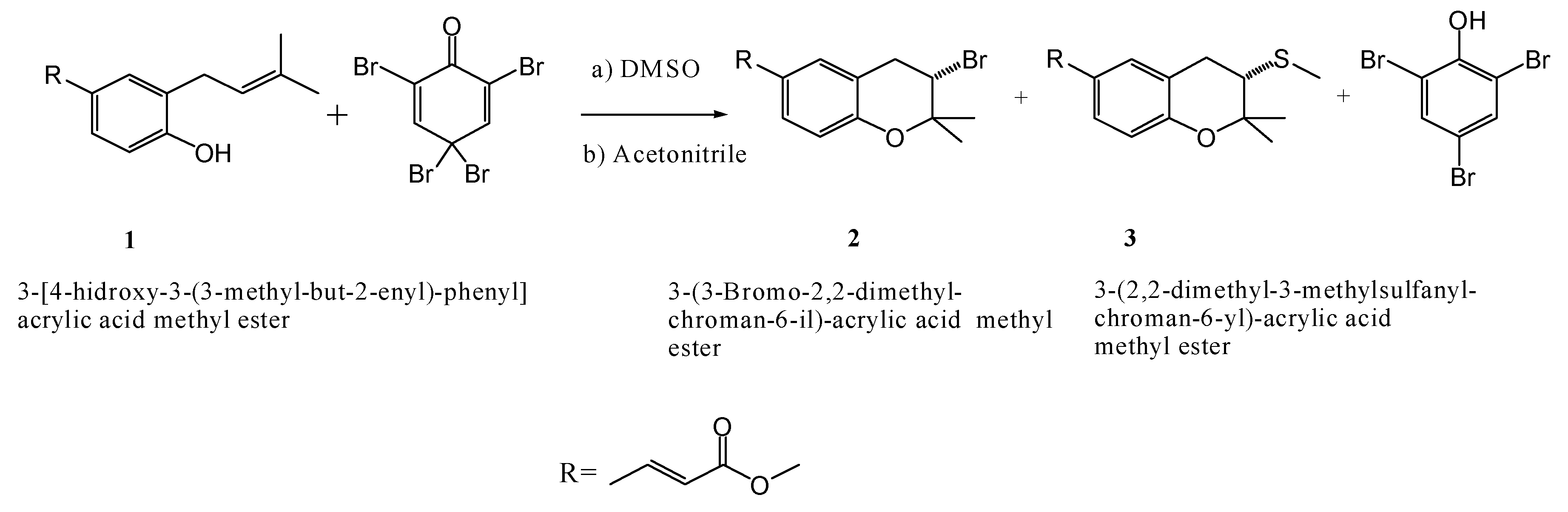
Reaction Mechanism for the Cyclization of 3-[γ,γ-Dimethylallyl]Coumaric Acid Methyl Ester in Dimethyl Sulfoxide (DMSO)
Introduction
Experimental
Results and Discussion

Acknowledgements
References and Notes
- Corey, E.J.; Kim, C.U. J. Am. Chem. Soc. 1972, 94, 7586. [CrossRef]
- Corey, E.J.; Kim, C.U. Tetrahedron Letters 1973, 919. [CrossRef]
- Lucchi, O. De; Miotti, U.; Modena, G. Organic Reactions; J. Wiley & Sons, Inc, 1991; Chapter 3; p. 40. [Google Scholar] [CrossRef]
Share and Cite
Borkowski, E.J.; Ardanaz, C.E.; Rossomando, P.C.; Tonn, C.E. Reaction Mechanism for the Cyclization of 3-[γ,γ-Dimethylallyl]Coumaric Acid Methyl Ester in Dimethyl Sulfoxide (DMSO). Molecules 2000, 5, 612-613. https://doi.org/10.3390/50300612
Borkowski EJ, Ardanaz CE, Rossomando PC, Tonn CE. Reaction Mechanism for the Cyclization of 3-[γ,γ-Dimethylallyl]Coumaric Acid Methyl Ester in Dimethyl Sulfoxide (DMSO). Molecules. 2000; 5(3):612-613. https://doi.org/10.3390/50300612
Chicago/Turabian StyleBorkowski, E. J., C. E. Ardanaz, P. C. Rossomando, and C. E. Tonn. 2000. "Reaction Mechanism for the Cyclization of 3-[γ,γ-Dimethylallyl]Coumaric Acid Methyl Ester in Dimethyl Sulfoxide (DMSO)" Molecules 5, no. 3: 612-613. https://doi.org/10.3390/50300612



