Thermolysis of Semicarbazones to the Corresponding Azines Through Reactive N-Substituted Isocyanate Intermediates
Abstract
:Introduction
Results and Discussion

- (Ia)
- undergoes π2s + π2a cycloaddition [17] giving the relatively unstable isocyanate dimer (Ib).
- (Ib)
- undergoes a threefold extrusion (2CO, N2) to give benzalazine (II).
Conclusion
Experimental
General
General Procedure for the Preparation of Azines (B) from Semicarbazones (A)
Spectral and Melting Point Data
Benzalazine 1B
p-Chlorobenzalazine 2B
p-Tolualdazine 3B
p-Nitrobenzalazine 4B
Bis(diethylamino)benzalazine 5B
3,4-Dimethoxybenzalazine 6B
Cyclohexanoneketazine 7B
Synthesis of 1-2 adducts (trapped products) 8, 9 and 10
Spectral and Melting Point Data
Benzylidene-N-decyl carbamate 8
4-n-Nonyloxy-benzylidene-N-decyl carbamate 9
[(4’-n-Dodecyloxy-benzaldehyde)- N-phenyl-(4-n-methoxy)] semicarbazone 10
Acknowledgements
References and Notes
- Reichen, W. Oxygen-, nitrogen- and sulfur-substituted heteroallenes. Chem. Rev. 1978, 78, 569–588. [Google Scholar] [CrossRef]
- Ulrich, H. Cycloaddition reactions of heterocumulenes. Academic Press: New York, 1967; Vol. 9. [Google Scholar]
- Lieber, E.; Minnis, R.L.; Rao, C.N.R. Carbamoyl azides. Chem. Rev. 1965, 65, 377–384. [Google Scholar] [CrossRef]
- Scotta, K.H.; Lornez, L. Uber Isocyanate, I.: Darstellung aliphatischer isocyanate. Ber. 1925, 58, 1320. [Google Scholar]
- Paraskeewas, S.M.; Panopolous, A.A. Isocyanates from isothiocyanates by oxidation with palladium (II) chloride and carbonic acid. Synthesis 1983, C8, 638–640. [Google Scholar]
- Ulrich, H. Chemistry and technology of isocyanates. John Wiley & Sons, 1996. [Google Scholar]
- Chudgar, N.K.; Shah, S.N.; Vora, R.A. Mesogenic semicarbazones and amino oxadiazoles-I. Mol. Cryst. Liq. Cryst. 1989, 172, 51–56. [Google Scholar] [CrossRef]
- Chudgar, N.K.; Shah, S.N.; Bapat, S. Synthesis of semicarbazones, amino-oxadiazoles and evaluation of their different properties. J. Inst. Chem. (India) 1990, 62, 185–186. [Google Scholar]
- Chudgar, N.K.; Shah, S.N. Mesogenic semicarbazones and amino oxadiazoles–II. J. M. S. University of Baroda 1993, 63–69. [Google Scholar]
- Kodaka, M.; Shah, S.N.; Tomohiro, T.; Chudgar, N.K. Theoretical analysis of mesogenic properties of benzalazines and benzopyran derivatives. J. Phys. Chem. B 1998, 102, 1219–1223. [Google Scholar] [CrossRef]
- Chudgar, N.K.; Shah, V.; Shah, S.N. Synthesis and characterization of new mesogenic phenylhydrazones. Mol. Mat. 1992, 1, 307–312. [Google Scholar]
- Shah, S.N. Synthesis and study of the new liquid crystalline 2,4 dinitrophenyl hydrazone derivative. J. Mys. Uni. Sect. B. 1994, 33A, 55–60. [Google Scholar]
- Chudgar, N.K.; Shah, S.N.; Vora, R.A. Mesogenic properties as an analytical tool for the pyrolytic transformation of substituted aryl aldehyde semicarbazone to corresponding benzalazine. Mol. Cryst. Liq. Cryst. 1991, 209, 237–241. [Google Scholar] [CrossRef]
- (a) Hoffman, H.; Diehr, H.P. Reactions of N-carbonylsulfamyl chloride with dienes. Tetrahedron Lett. 1963, 1875. [Google Scholar] (b) Hoffman, H.; Diehr, H.P. Einwirkung von N-carbonyl-sulfamisaurechlorid auf diene. Angew Chem. 1964, 3, 649. [Google Scholar]
- Perkin, W.H., Jr.; Plant, S.G.P. 1,2,3,4,5,6,7,8-octahydrocarbazole and its derivatives. J. Chem. Soc. 1924, 125, 1503–1513. [Google Scholar] [CrossRef]
- Nakata, H.; Tatematsu, A. Behavior of semicarbazones in a mass spectrometer. Chem. Commun. 1967, 5, 208–209. [Google Scholar]
- Brown, C.J. Dimerization of isocyanate is well documented in the literature. The crystal structure of the phenyl isocyanate dimer. J. Chem. Soc. 1955, 2931–2936. [Google Scholar] [CrossRef]
- Shapiro, N. Preparation of azines by means of hydrazines hydrochloride. Ber. 1933, 66B, 1103–1107. [Google Scholar]
- Bogoslovskil, B.M.; Yakovenko, T.I. Benzalazine and its derivatives. I. Action of hydrogen chloride on benzalazine and its derivatives. Zh. Obshchei. Khim. 1957, 27, 159–168. [Google Scholar]
- Pascal, P.; Normand, L. Decomposition of azines by heat. Bull. Soc. Chim. 1911, 9, 1059–1068. [Google Scholar]
- Howard, L.B.; Hilbert, G.E.; Wiebe, W.R.; Gaddy, V.L. The thermal decomposition of azines. J. Am. Chem. Soc. 1932, 54, 3628–3641. [Google Scholar] [CrossRef]
- Barany, H.C.; Braude, E.A.; Pianka, M. Studies in light absorption. Part VII. Azines and related systems. A comparison of the –C=C- and –C=N- chromophore. J. Chem. Soc. 1949, 1898–1902. [Google Scholar] [CrossRef]
- Egorova, V.I. Catalytic hydrogenation of alicyclic hydrazines. I. Hydrogenation of ketazines of cyclohexanone and its methyl derivatives. J. Gen. Chem. (U.S.S.R) 1936, 6, 1404–1417. [Google Scholar]
- Samples Availability: Available from the authors.
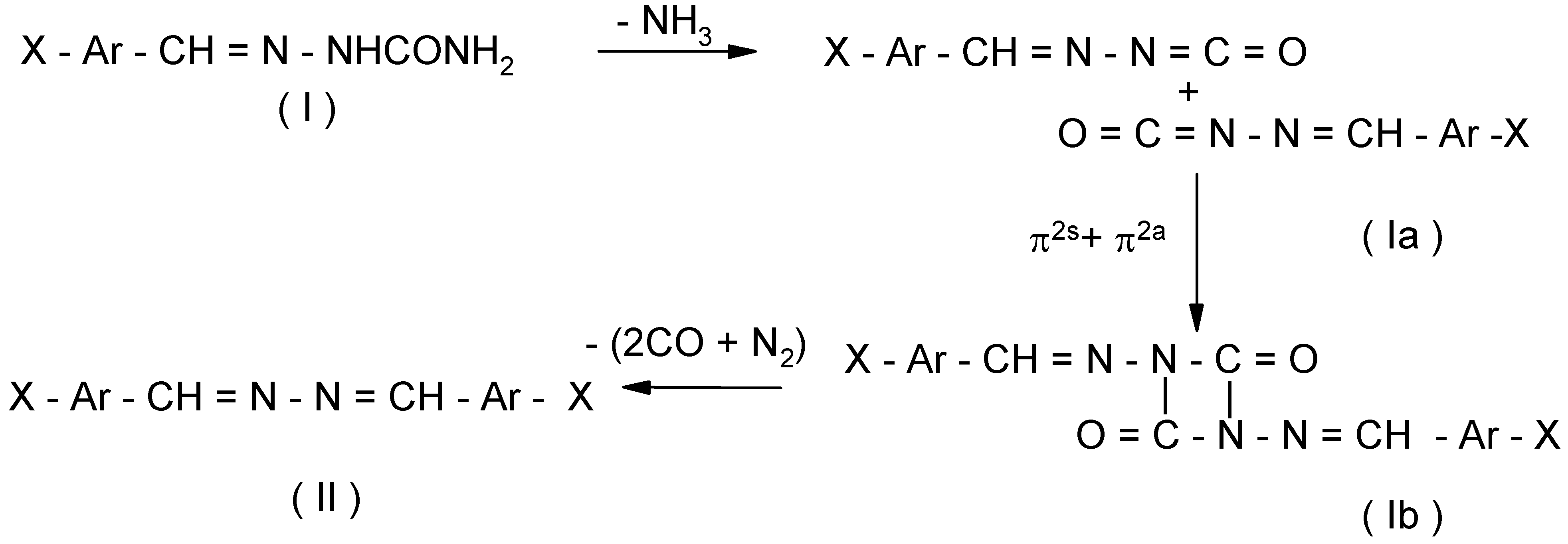
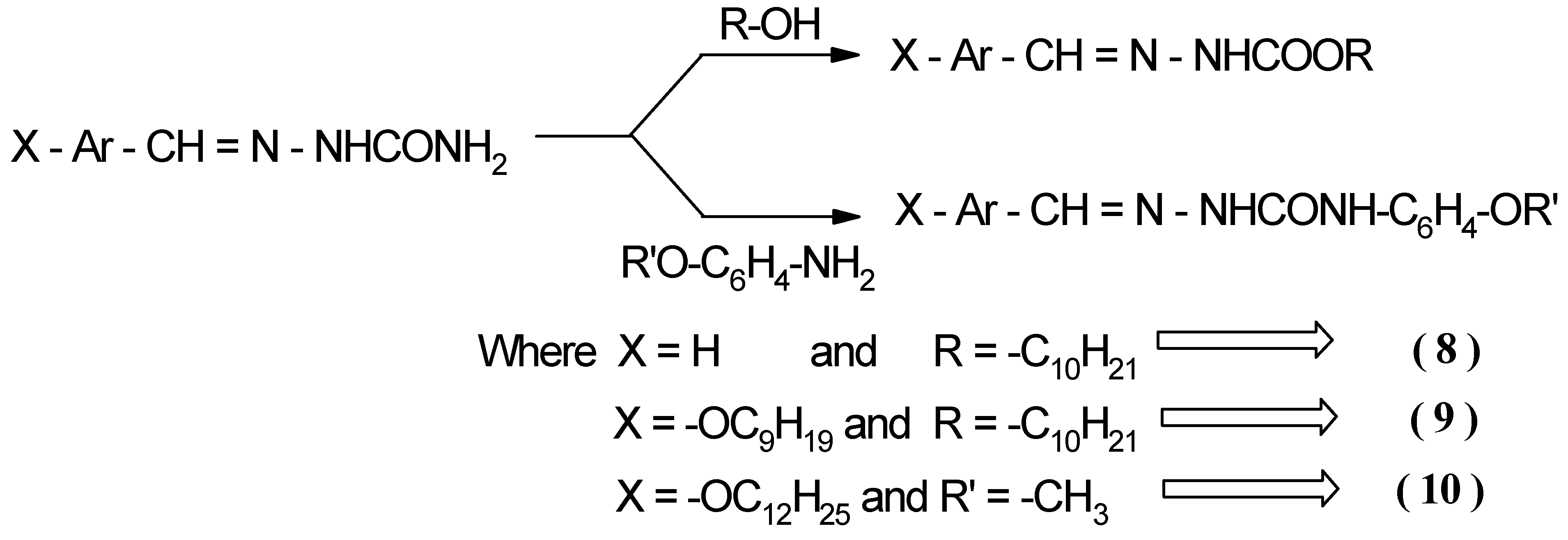
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Shah, S.N.; Chudgar, N.K. Thermolysis of Semicarbazones to the Corresponding Azines Through Reactive N-Substituted Isocyanate Intermediates. Molecules 2000, 5, 657-664. https://doi.org/10.3390/50400657
Shah SN, Chudgar NK. Thermolysis of Semicarbazones to the Corresponding Azines Through Reactive N-Substituted Isocyanate Intermediates. Molecules. 2000; 5(4):657-664. https://doi.org/10.3390/50400657
Chicago/Turabian StyleShah, S. N., and N. K. Chudgar. 2000. "Thermolysis of Semicarbazones to the Corresponding Azines Through Reactive N-Substituted Isocyanate Intermediates" Molecules 5, no. 4: 657-664. https://doi.org/10.3390/50400657





