Experimental
General
All melting points are uncorrected. The 1H-NMR and 13C-NMR spectra were recorded on Bruker (WM-250 MHz), Bruker (AC-250 MHz) spectrometers (Faculty of Chemistry, Konstanz University, Germany), and a Varian 1H Gemini 200 spectrometer (National Research Center, Egypt) and chemical shifts were expressed as δ values against SiMe4 used as internal standard. IR spectra were recorded as potassium bromide pellets on a Perkin-Elmer 1430 spectrometer, (National Research Center and De-partment of Chemistry, Cairo University) and Perkin-Elmer 1320 and 299 spectrometers (Faculty of Chemistry, Konstanz University, Germany). Mass spectra were recorded on a Shimadzu (Japan) GCMS-QP 1000 EX gas chromatography-mass spectrometer system. Microanalytical data were ob-tained by the Microanalytical Center at Konstanz University (Germany), Cairo University and National Research Center (Egypt).
General procedure for the preparation of 3a,b
A mixture of compound
2a or
2b [2] (10 mmol), formic acid (10 mL) and catalytic amount of con-centrated hydrochloric acid was heated under reflux for 5 h. The reaction mixture was allowed to cool to room temperature and poured into water (100 mL). The formed solid was collected by filtration, washed with ethanol, dried and crystallized from the proper solvent.
4,6,7-Trimethyl-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (3a)
From 2a, crystallized from dioxane (50 mL) in 73% yield, m.p. 267-9°C; IR (KBr) cm-1: 2980 (CH alkyl), 1689 (C=O), 1576 (C=N), 1519 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.55 (s,6H,2CH3), δ 3.86 (s,3H,N-CH3), δ 8.58 (s,1H, methylenic proton); 13C-NMR (DMSO-d6): 13.22, 13.29 (2CH3), 31.19 (N-CH3) 122.85-148.50 (6C, sp2 carbon atoms), 157.55 (C=O). Analyses: C10H10N4OS (234.3). Re-quired: C, 51.27; H, 4.31; N, 23.92; Found: C, 51.19; H, 4.28; N, 23.64.
4-Methyl-6,7,8,9-tetrahydrobenzo-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (3b)
From 2b, crystalized from dioxane (40 ml) in 76% yield, m.p. 243-45°C; IR (KBr) cm-1: 2983 (CH alkyl), 1669 (C=O), 1581 (C=N), 1509 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.92 (m, 4H, 2CH2), δ 2.78 (t, 2H, CH2), δ 3.00 (t, 2H, CH2), δ 3.73 (s, 3H, N-CH3), δ 8.40 (s, 1H, methylenic proton); 13C-NMR (DMSO-d6): 21.83-25.53 (4CH2), 29.19 (N-CH3) 118.34-148.96 (6C, sp2 carbon atoms), 156.30 (C=O). Analyses: C12H12N4OS (260.3). Required: C, 55.37; H, 4.66; N, 21.53; Found: C, 55.19; H, 4.52; N, 20.98.
General procedure for the preparation of 3c,d
A mixture of 2a or 2b (10 mmol) and glacial acetic acid (30 mL) was stirred under reflux for 18 hr. The reaction mixture was allowed to cool to room temperature and poured into water (100 mL). The solid thus formed was collected by filtration, washed with ethanol (20 mL), dried, and crystallized from acetic acid.
1,4,6,7-Tetramethyl-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (3c)
From 2a in 69% yield, m.p. 226-29°C; IR (KBr) cm-1: 2980 (CH alkyl), 1650 (C=O), 1580 (C=N), 1500 (C=C); 1H-NMR (TFA:CDCl3/1:1) ppm: δ 2.55 (s, 6H, 2CH3), δ 3.08 (s, 3H, CH3), δ 3.76 (s, 3H, N-CH3); 13C-NMR: 11.22, 12.97, 13.05 (3CH3), 30.50 (N-CH3) 122.16-148.55 (6C sp2 carbon atoms), 157.28 (C=O). Analyses: C11H12N4OS (248.3). Required: C, 53.21; H, 4.88; N, 22.57; Found: C, 53.15; H, 4.49; N, 22.2.
1,4-Dimethyl-6,7,8,9-tetrahydrobenzo-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (3d)
From 2b in 73% yield, m.p. 238-41°C; IR (KBr) cm-1: 2956 (CH alkyl), 1687 (C=O), 1562 (C=N), 1522 (C=C); 1H-NMR (TFA:CDCl3/1:1) ppm: δ 1.96 (m, 4H, 2CH2), δ 2.22 (s, 3H, CH3), δ 2.93 (t, 2H, CH2), δ 3.09 (t, 2H, CH2), δ 3.77 (s, 3H, N-CH3); Analyses: C13H14N4OS (274.4). Required: C, 56.92; H, 5.15; N, 20.42; Found: C, 56.81; H, 5.21; N, 20.13.
General procedure for the preparation of 4a,b
A solution of sodium nitrite (1.04g, 15 mmol) in the least amount of water was added dropwise to an an ice-cold solution of compound 2a or 2b (10 mmol) in acetic acid (10 mL) kept an ice bath at –5°C. The reaction mixture was allowed to stand overnight at room temperature, then it was poured into wa-ter (100 mL). The solid so-precipitated was filtered off and crystallized from benzene.
4,6,7-Trimethyl-4,5-dihydrothieno[3,2-e][1,2,3,4]tetrazolo[1,5-a]pyrimidin-5-one (4a)
From 2a in 53% yield, m.p. 183-85°C (dec.); IR (KBr) cm-1: 2954 (CH alkyl), 1713 (C=O), 1629 (N=N), 1582 (C=N), 1506 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.47 (s, 6H, 2CH3), δ 3.88 (s, 3H, N-CH3); 13C-NMR: 11.22, 12.97 (2CH3), 30.53 (N-CH3) 118.22-148.55 (5C, sp2 carbon atoms), 157.28 (C=O). Analyses: C9H9N5OS (235.3). Required: C, 45.94; H, 3.86; N, 29.77; Found: C, 45.81; H, 3.65; N, 30.05.
4-Methyl-6,7,8,9-tetrahydrobenzo-4,5-dihydrothieno[3,2-e][1,2,3,4]tetrazolo[1,5-a]pyrimidin-5-one (4b)
From 2b in 61% yield, m.p. 210-12°C (dec.); IR (KBr) cm-1: 2943 (CH alkyl), 1698 (C=O), 1609 (N=N), 1567 (C=N), 1520 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.97 (m, 4H, 2CH2), δ 2.95 (t, 2H, CH2), δ 3.19 (t, 2H, CH2), δ 3.82 (s, 3H, N-CH3); Analyses: C11H11N5OS (261.3). Required: C, 50.56; H, 4.25; N, 26.81; Found: C, 50.32; H, 4.11; N, 27.01.
General procedure for the preparation of 4`a,b
Activated zinc dust (5.00g) was added protionwise to a well stirred solution the appropriate tetra-zolo-thienopyrimidine 4a or 4b (10 mmol) in glacial acetic acid (30 mL) at room temperature over a period of 30 minutes. Stirring was continued for additional 3 hr. Then the reaction mixture was heated on a water bath (80-90°C) for 3 hr. The progress of reduction was monitored by TLC. After allowing the reaction mixture to cool to room temperature, it was poured into cold water (100 mL). The insolu-ble solid which separated was filtered, washed with water and dried. The crude solid was extracted with hot benzene and the solid obtained after removal of benzene was crystallized from acetic acid.
2-Amino-3,5,6-trimethyl-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (4`a)
From 4a, in 49% yield, m.p. 321-24°C; IR (KBr) cm-1: 3280 (brs, NH2), 2923 (CH alkyl), 1686 (C=O), 1593 (C=N), 1557 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.07 (s, 3H, CH3), δ 2.31 (s, 3H, CH3), δ 3.88 (s, 3H, N-CH3), δ 3.25 (brs, 2H, NH2, D2O exchangeable); 13C-NMR: 11.24, 12.96 (2CH3), 30.54 (N-CH3), 118.3-148.61 (5C sp2 carbon atoms), 160.12 (C=O). Analyses: C9H11N3OS (209.3). Required: C, 51.64; H, 5.31; N, 20.08; Found: C, 51.49; H, 5.17; N, 19.79.
Amino-3-methyl-5,6,7,8-tetrahydrobenzo-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (4`b)
From 4b, in 51% yield, m.p. 298-301°C; R (KBr) cm-1: 3245 (brs, NH2), 2953 (CH alkyl), 1694 (C=O), 1560 (C=N), 1541 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.94 (m, 4H, 2CH2), δ 2.91 (t, 2H, CH2), δ 3.17 (t, 2H, CH2), δ 3.87 (s, 3H, N-CH3), 10.50 (brs, 2H, NH2, D2O exchangeable). Analyses: C11H13N3OS (235.3). Required: C, 56.15; H, 5.58; N, 17.86; Found: C, 55.98; H, 5.39; N, 17.91.
General procedure for the preparation of 5a,b
To a warmed ethanolic sodium hydroxide solution prepared by dissolving of sodium hydroxide (0.40g, 10 mmol) in ethanol (50 mL) was added (10 mmol) of compound (2a,b) and excess carbon di-sulphide (10 mL). The mixture was refluxed in a water bath at 80°C for 10 hr, then allowed to cool to room temperature, poured into water (100 mL), neutralized by dilute acetic acid and the precipitate formed was filtered off and dried. The product was crystallized from benzene.
4,6,7-Trimethyl-1-mercapto-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (5a)
From 2a in 71% yield, m.p. 266-68°C (dec.); IR (KBr) cm-1: 2944 (CH alkyl), 1676 (C=O), 1635 (C=N), 1583 (C=C) ; 1H-NMR (DMSO-d6) ppm: δ 2.17 (s, 3H, CH3), δ 2.22 (s, 3H, CH3), δ 2.44 (s, 1H, SH), δ 3.97 (s, 3H, N-CH3). Analyses: C10H10N4OS2 (266.4). Required: C, 45.10; H, 3.79; N, 21.04; Found: C, 45.33; H, 3.61; N, 21.21.
4-Methyl-1-mercapto-6,7,8,9-tetrahydrobenzo-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimi-din-5-one (5b)
From 2b in 71% yield, m.p. 233-35°C (dec.); IR (KBr) cm-1:, 2931 (CH alkyl), 1680 (C=O), 1632 (C=N), 1571 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.98 (m, 4H, 2CH2), δ 2.53 (s, 1H, SH), δ 2.94 (t, 2H, CH2), δ 3.09 (t, 2H, CH2), δ 3.94 (s, 3H, N-CH3). Analyses: C12H12N4OS2 (292.4). Required: C, 49.29; H, 4.15; N, 19.17; Found: C, 49.20; H, 4.18; N, 19.28.
General procedure for the preperation of 6a-f
A mixture of compound 2a or 2b (10 mmol), the appropriate aromatic aldehyde (10 mmol) and an-hydrous sodium acetate (1.64g, 20 mmol) was stirred under reflux in glacial acetic acid (30 mL) for 5 hr. The reaction mixture was allowed to cool to room temperature, poured into water (100 mL), whereby the formed solid was filtered off and crystallized from an appropriate solvent to produce 6a-f in high yields.
3,5,6-Trimethyl-3,4-dihydrothieno[2,3-d]pyrimidin-4-one-2-benzaldehyde hydrazone (6a)
From compound 2a (10 mmol) and benzaldehyde (1.06g, 10 mmol). The compound was obtained as pale white crystals, crystallized from acetic acid in 70% yield, m.p. 288-91°C; IR (KBr) cm-1: 3373 (brs, NH), 3047 (CH aryl), 2917 (CH alkyl), 1675 (C=O), 1625 (C=N), 1586 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.22 (s, 3H, CH3), δ 2.31 (s, 3H, CH3), δ 3.84 (s, 3H, N-CH3), δ 7.21-7.75 (m,5H,phenyl pro-tons), δ 8.89 (s,1H,methylenic proton), δ 11.52 (br,1H,NH,D2O exchangeable); Analyses: C16H16N4OS (312.4) Requierd: C, 61.51; H, 5.16; N, 17.94; Found: C, 61.47; H, 4.99; N, 18.01.
3,5,6-Trimethyl-3,4-dihydrothieno[2,3-d]pyrimidin-4-one-2-p-chlorobenzaldehyde hydrazone (6b)
From compound 2a (10 mmol) and 4-chlorobenzaldehyde (1.41g, 10 mmol). The compound was obtained as pale light yellow crystals, crystallized from dioxane in 72% yield, m.p. 278-80°C (dec.); IR (KBr) cm-1: 3250 (br, NH), 3040 (CH aryl), 2920 (CH alkyl), 1670 (C=O), 1600 (C=N), 1500 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.23 (s, 3H, CH3), δ 2.31 (s, 3H, CH3), δ 3.89 (s, 3H, N-CH3) δ 7.62 (dd, 4H, phenyl protons), δ 8.84 (s, 1H, methylenic proton), δ 14.02 (brs, 1H, NH, D2O exchangeable); 13C-NMR: 12.06, 12.56 (2CH3), 30.42 (N-CH3) 119.89-158.35 (12C, sp2 carbon atoms), 164.38 (C=O). Analyses: C16H15ClN4OS (346.9). Required: C, 55.40; H, 4.37; N, 16.15; Found: C, 55.21; H, 4.28; N, 16.32.
3,5,6-Trimethyl-3,4-dihydrothieno[2,3-d]pyrimidin-4-one-2-p-methoxybenzaldehyde hydrazone (6c)
From compound 2a (10 mmol) and 4-methoxybenzaldehyde (1.36g, 10 mmol). The compound was obtained as pale white crystals, crystallized from dioxane in 68% yield, m.p. 246-49°C (dec.); IR (KBr) cm-1: 3368 (brs, NH), 3044 (CH aryl), 2916 (CH alkyl), 1676 (C=O), 1605 (C=N), 1517 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.25 (s, 3H, CH3), δ 2.30 (s, 3H, CH3), δ 3.79 (s, 3H, N-CH3), δ 3.93 (s, 3H, OCH3), δ7.41 (dd, 4H, phenyl protons), δ 7.98 (s, 1H, methylenic proton), δ 11.48 (brs, 1H, NH, D2O exchangeable). Analyses: C17H18N4O2S (342.5). Required: C, 59.61; H, 5.31; N, 16.38; Found: C, 59.37; H, 5.41; N, 16.19.
3-Methyl-5,6,7,8-tetrahydrobenzo-3,4-dihydrothieno[2,3d]pyrimidin-4-one-2-benzaldehyde hydrazone (6d)
From compound 2b (10 mmol) and benzaldehyde (1.06g, 10 mmol). The compound was obtained as pale white crystals, crystallized from acetic acid in 71% yield, m.p. 242-44°C; IR (KBr) cm-1: 3370 (brs, NH), 3047 (CH aryl), 2919 (CH alkyl), 1674 (C=O), 1615 (C=N), 1596 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.92 (m, 4H, 2CH2), δ 2.91 (t, 2H, CH2), δ 3.09 (t, 2H, CH2), δ 3.94 (s, 3H, N-CH3), δ 7.09-7.85 (m, 5H, phenyl protons), δ 8.45 (s, 1H, methylenic proton), δ 12.20 (brs, 1H, NH, D2O exchange-able). Analyses: C18H18N4OS (338.5). Requierd: C, 63.87; H, 5.37; N, 16.56; Found: C, 63.76; H, 5.19; N, 16.28.
3-Methyl-5,6,7,8-tetrahydrobenzo-3,4-dihydrothieno[2,3-d]pyrimidin-4-one-2-(p-chlorobenzalde-hyde hydrazone (6e)
From compound 2b (10 mmol) and 4-chlorobenzaldehyde (1.41g, 10 mmol). The compound was obtained as pale light yellow crystals, crystallized from dioxane in 72% yield, m.p. 255-58°C (dec.); IR (KBr) cm-1: 3250 (brs, NH), 3040 (CH aryl), 2921 (CH alkyl), 1673 (C=O), 1609 (C=N), 1524 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.96 (m, 4H, 2CH2), δ 2.94 (t, 2H, CH2), δ 3.05 (t, 2H, CH2), δ 3.94 (s, 3H, N-CH3), δ 7.65 (dd, 4H, phenyl protons), δ 8.44 (s, 1H, methylenic proton), δ 10.67 (brs, 1H, NH, D2O exchangeable); 13C-NMR: 21.77-25.59 (4CH2), 30.19 (N-CH3) 118.54-158.89 (12C, sp2 carbon atoms), 161.35 (C=O). Analyses: C18H17N4ClOS (372.9). Required: C, 57.97; H, 4.60; N, 15.03; Found: C, 57.81; H, 4.47; N, 14.79.
3-Methyl-5,6,7,8-tetrahydrobenzo-3,4-dihydrothieno[2,3-d]pyrimidin-4-one-2-(p-methoxybenzalde-hyde hydrazone (6f)
From compound 2b (10 mmol) and 4-methoxybenzaldehyde (1.36g, 10 mmol). The compound was obtained as pale light white crystals, crystallized from dioxane in 68% yield, m.p. 262-64°C (dec.); IR (KBr) cm-1: 3368 (brs, NH), 3044 (CH aryl), 2916 (CH alkyl), 1676 (C=O), 1605 (C=N), 1517 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.93 (m, 4H, 2CH2), δ 2.97 (t, 2H, CH2), δ 3.01 (t, 2H, CH2), δ 3.83 (s, 3H, N-CH3), δ 3.95 (s, 3H, OCH3), δ 7.42 (dd, 4H, phenyl protons), δ 8.08 (s, 1H, methylenic proton), δ 11.25 (brs, 1H, NH, D2O exchangeable). Analyses: C19H20N4O2S (368.5). Required: C, 61.93; H, 5.48; N, 15.21; Found: C, 61.88; H, 5.31; N, 15.7.
General procedure for the preparation of 7a-f
A mixture of compound 6a-f (10 mmol), anhydrous sodium acetate (1.64g, 20 mmol) and bromine (1.60g, 10 mmol) was heated gently in glacial acetic acid (30 mL) in a water bath at 80°C for 16 hr. The reaction mixture was allowed to cool to room temperature, poured into water (100 ml) and the solid so-formed was collected by filtration and crystallized from appropriate solvent, to yield 7a-c.
1-Phenyl-4,6,7-trimethyl-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidinone (7a)
From compound 6a (10 mmol). The compound was obtained as yellow crystals, crystallized from di-oxane in 62% yield, m.p. 328-30°C (dec.); IR (KBr) cm-1: 3049 (CH aryl), 2914 (CH alkyl), 1653 (C=O), 1556 (C=N), 1489 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.22 (s, 3H, CH3), δ 2.35 (s, 3H, CH3), δ 3.89 (s, 3H, N-CH3) δ 7.09-7.44 (m, 5H, phenyl protons); 13C-NMR (DMSO-d6) ppm: 12.61, 12.83, 31.41 (3CH3), 118.26-159.24 (12C sp2 carbon atoms), 162.91 (C=O). Analyses: C16H14N4OS (310.4). Required: C, 61.91; H, 4.56; N, 18.05; Found: C, 61.72; H, 4.41; N, 18.17.
1-(4-Chlorophenyl)-4,6,7-trimethyl-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (7b)
From compound 6b (10 mmol). The compound was obtained as pale yellow crystals, crystallized from dioxane in 59% yield, m.p. 302-4°C (dec.); IR (KBr) cm-1: 3060 (CH aryl), 2920 (CH alkyl), 1687 (C=O), 1611 (C=N), 1556 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.35 (s, 3H, CH3), δ 2.45 (s, 3H, CH3), δ 3.85 (s, 3H, N-CH3), δ 7.65 (dd, 4H, phenyl protons); Analyses: C16H13ClN4OS (344.8). Required: C, 55.73; H, 3.81; N, 16.25; Found: C, 55.59; H, 3.71; N, 16.03.
1-(4-Methoxyphenyl)-4,6,7-trimethyl-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (7c)
From compound 6c (10 mmol). The compound was obtained as light yellow crystals, crystallized di-oxane in 48% yield, m.p. 282-84°C (dec.); IR (KBr) cm-1: 3046 (CH aryl), 2916 (CH alkyl), 1664 (C=O), 1557 (C=N), 1499 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.33 (s, 3H, CH3), δ 2.41 (s, 3H, CH3), δ 3.89 (s, 3H, N-CH3), δ 7.14 (dd, 4H, phenyl protons); Analyses: C17H16N4O2S (340.4). Required: C, 59.98; H, 4.75; N, 16.46; Found: C, 59.73; H, 4.52; N, 16.28.
4-Methyl-1-phenyl-6,7,8,9-tetrahydrobenzo-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-b]pyrimi-din-5-one (7d)
From compound 6d (10 mmol). The compound was obtained as yellow crystals, crystallized from di-oxane in 52% yield, m.p. 272-74°C (dec.); IR (KBr) cm-1: 3049 (CH aryl), 2914 (CH alkyl), 1653 (C=O), 1556 (C=N), 1489 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.94 (m, 4H, 2CH2), δ 2.90 (t, 2H, CH2), δ 3.19 (t, 2H, CH2), δ 3.91 (s, 3H, N-CH3), δ 7.21-7.56 (m, 5H, phenyl protons); 13C-NMR: 21.83-25.53 (4CH2), 29.89 (N-CH3) 118.34-158.96 (12C, sp2 carbon atoms), 163.30 (C=O); Analyses: C18H16N4OS (336.4). Required: C, 64.26; H, 4.80; N, 16.66; Found: C, 64.02; H, 4.63; N, 16.49.
4-Methyl-1-(4-chlorophenyl)-6,7,8,9-tetrahydrobenzo-4,5-dihydrothieno[3,2-e][1,2,4triazolo[3,4-a]-pyrimidin-5-one (7e)
From compound 6e (10 mmol). The compound was obtained as pale yellow crystals, crystallized from dioxane in 59% yield, m.p. 287-90°C (dec.); IR (KBr) cm-1: 3060 (CH aryl), 2920 (CH alkyl), 1653 (C=O), 1611 (C=N), 1556 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.92 (m, 4H, 2CH2), δ 2.93 (t, 2H, CH2), δ 3.11 (t, 2H, CH2), δ 3.86 (s, 3H, N-CH3), δ 7.41 (dd, 4H, phenyl protons). Analyses: C18H15ClN4OS (370.9). Required: C, 58.29; H, 4.09; N, 15.11; Found: C, 58.22; H, 3.91; N, 14.98.
4-Methyl-1-(4-methoxyphenyl)-6,7,8,9-tetrahydrobenzo-4,5-dihydrothieno[3,2-e][1,2,4]triazolo[3,4-a]pyrimidin-5-one (7f)
From compound 6f (10 mmol). The compound was obtained as a light yellow crystals, crystallized from dioxane in 51% yield, m.p. 301-303°C (dec.); IR (KBr) cm-1: 3054 (CH aryl), 2916 (CH alkyl), 1664 (C=O), 1557 (C=N), 1499 (C=C). Analyses: C19H18N4SO2 (366.5). Required: C, 62.26; H, 4.96; N, 15.29; Found: C, 62.17; H, 4.88; N, 15.09.
General procedure for the preparation of 8a,b
A mixture of compound 2a or 2b (10 mmol) and ethyl acetoacetate (1.30g, 10 mmol) was refluxed in absolute ethanol (30 mL) for 5 hr. The reaction mixture was allowed to cool and the solid product so produced was filtered off and crystallized from ethanol to produce 8a or 8b.
Ethylacetoacetatehydrazone-3,5,6-trimethyl-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (8a)
From 2a in 85% yield, m.p. 149-52°C ; IR (KBr) cm-1: 3156 (brs, NH), 2949 (CH alkyl), 1730, 1672 (2C=O), 1567 (C=N), 1511 (C=C); 1H-NMR (CDCl3) ppm: δ 1.27 (t, 3H, CH3), δ 2.00 (s, 3H, CH3), δ 2.28 (s, 3H, CH3), δ 2.40 (s, 3H, CH3), δ 3.26 (s, 2H, CH2), δ 3.89 (s, 3H, N-CH3) δ 4.19 (q, 2H, CH2), δ 9.39 (brs, 1H, NH, D2O exchangeable); 13C-NMR: 12.57, 12.96, 14.15, 15.77, 30.23 (5CH3), 44.32, 61.29 (2CH2), 118.08-158.37 (6C sp2 carbon atoms), 164.72, 169.31 (2C=O). Analyses: C15H20N4O3S (336.5). Required: C, 53.54; H, 5.99; N, 16.65; Found: C, 53.39; H, 5.67; N, 16.47.
2-Ethylacetoacetatehydrazone-5,6,7,8-tetrahydrobenzo-3-methyl-3,4-dihydrothieno[2,3-d] pyrimidin-4-one (8b)
From 2b in 85% yield, m.p. 162-64°C; IR (KBr) cm-1: 3150 (brs, NH), 2960 (CH alkyl), 1740, 1680 (2C=O), 1580 (C=N), 1500 (C=C); 1H-NMR (CDCl3) ppm: δ 1.29 (t, 3H, CH3), δ 1.96 (m, 4H, 2CH2), δ 2.90 (t, 2H, CH2), δ 3.11 (t, 2H, CH2), δ 3.22 (s, 2H, CH2), δ 3.80 (s, 3H, N-CH3), δ 4.13(q, 2H, CH2), δ 10.31(brs, 1H, NH, D2O exchangeable); Analyses: C17H22N4SO3 (362.5). Required: C, 56.34; H, 6.13; N, 15.46; Found: C, 56.21; H, 6.06; N, 15.39.
General procedure for the preparation of 9a,b
Method (A): A solution of compound 2a or 2b (10 mmol) and ethyl acetoacetate (1.30g, 10 mmol) was stirred under reflux in absolute ethanol (30 mL) for 30 hr. The reaction mixture was allowed to cool to room temperature, poured into cold water (100 mL). The deposited precipitate was filtered off, dried and crystallized from dioxane.
Method (B): A solution of compound 2a or 2b (10 mmol) and ethyl acetoacetate (1.30g, 10 mmol) in sodium ethoxide solution [prepared by dissolving sodium metal (0.23g, 10 mmol) in absolute ethanol (30 mL)] was heated under reflux with stirring for 6 hr. The reaction mixture was allowed to cool and poured into cold water (100 mL) and neutralized by acetic acid, whereby a solid was precipitated, which was filtered off and crystallized from dioxane.
Method (C): A solution of compound 2a or 2b (10 mmol) was heated under reflux with sodium ethoxide solution [sodium metal (0.23g, 10 mmol) in absolute ethanol (30 ml)] for 3 hr. The reaction mixture was allowed to cool, poured into water (100 mL), neutralized with acetic acid, and the precipi-tate formed was filtered off and crystallized from dioxane.
5,6-Dimethyl-2-(3-methyl-4H,5H-pyrazol-5-one-1-yl)-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (9a)
From 8a, in 72% yield, m.p. 219-22°C ; IR (KBr) cm-1: 2940 (CH alkyl), 1688, 1666 (2C=O), 1550 (C=N), 1500 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.30-2.33 (m, 9H, 3CH3), δ 2.52 (s, 2H, CH2), δ 3.89 (s, 3H, N-CH3). Analyses: C13H14N4O2S (290.4). Required: C, 53.77; H, 4.87; N, 19.30; Found: C, 53.65; H, 4.52; N, 19.07.
5,6,7,8-Tetrahydrobenzo-2-(3-methyl-4H,5H-pyrazol-5-one-1-yl)-3-methylthieno[2,3-d]pyrimidin-4-one (9b)
From 8b, in 72% yield, m.p. 232-35°C; IR (KBr) cm-1: 2940 (CH alkyl), 1670, 1600 (2C=O), 1550 (C=N), 1500 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.91 (m, 4H, 2CH2), δ 2.23 (s, 3H, CH3), δ 2.52 (s, 2H, CH2), δ 2.90 (t, 2H, CH2), δ 3.11 (t, 2H, CH2), δ 3.90 (s, 3H, N-CH3); Analyses: C15H16N4O2S (316.4). Required: C, 56.94; H, 5.11; N, 17.71; Found: C, 56.88; H, 4.92; N, 17.56.
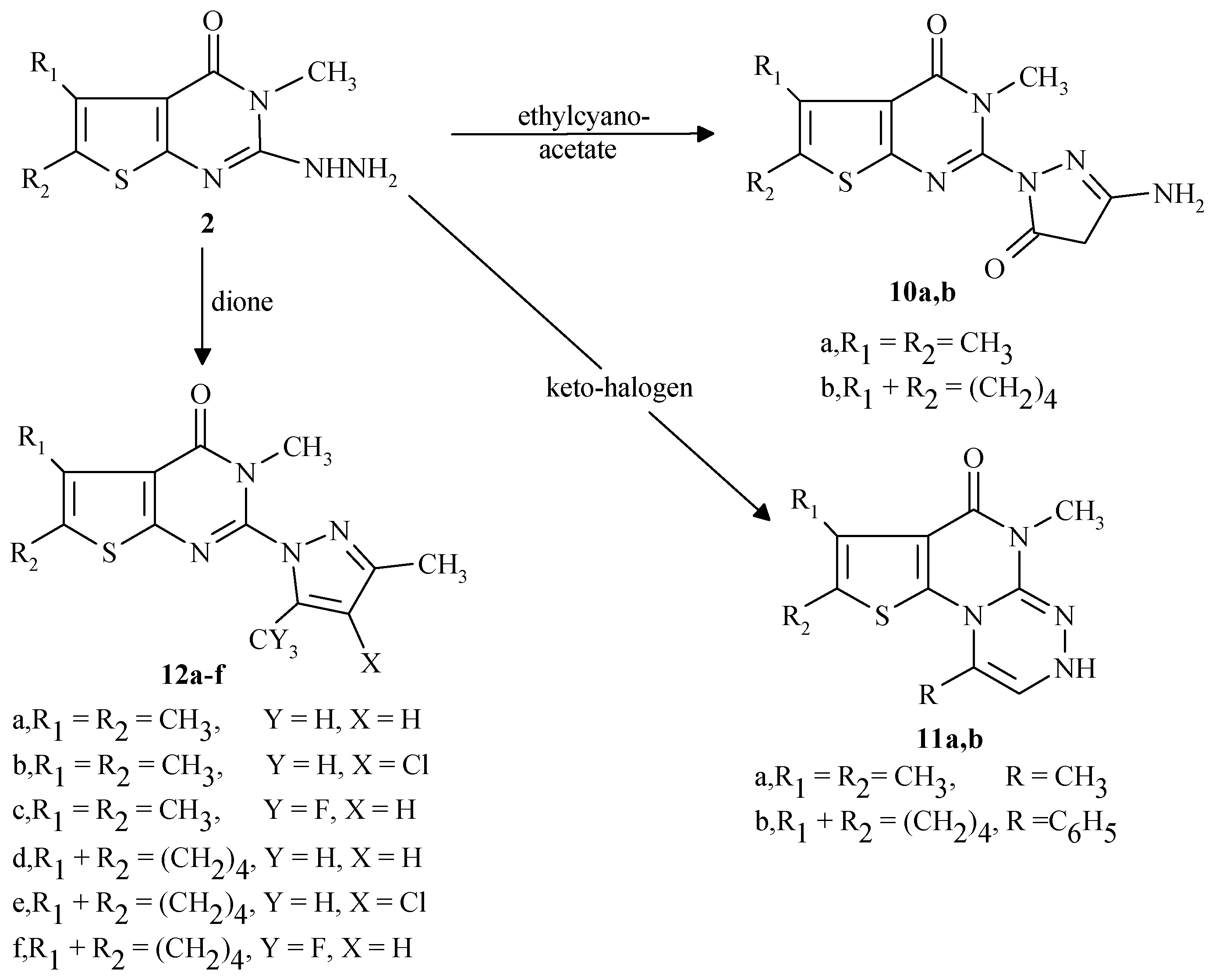
General procedure for the preparation of 10a,b
To a warmed ethanolic sodium ethoxide solution [prepared by dissolving sodium metal (0.23g, 10 mmol in absolute ethanol (30 mL)] was added either compound 2a or 2b (10 mmol) and ethyl cyano-acetate (1.13g, 10 mmol). The mixture was stirred under reflux for 8 hr, the reaction mixture was al-lowed to cool to room temperature, then poured into cold water (100 mL) and neutralized with acetic acid. The solid product was filtered off, washed with water, ethanol, dried and crystallized from diox-ane.
5,6-Dimethyl-2-(3-amino-4H,5H-5-pyrazolinon-1-yl)-3-methylthieno[2,3-d]pyrimidin-4-one (10a)
From 2a in 38% yield, m.p.334-7°C (dec.); IR (KBr) cm-1: 3265 (brs, NH), 2939 (CH alkyl), 1698 (C=O), 1609 (C=N), 1527 (C=C). 1H-NMR (DMSO-d6) ppm: δ 2.32 (s, 3H, CH3), δ 2.42 (s, 3H, CH3), δ 3.42 (s, 2H, CH2), δ 3.73 (s, 3H, N-CH3), δ 12.3 (brs, NH2, D2O exchangeable); 13C-NMR: 12.72 (CH3), 12.93 (CH3), 14.31 (CH2),37.58 (N-CH3), 118.01-153.27 (6C sp2 carbon atoms), 159.32, 161.23 (2C=O). Analyses: C12H13N5O2S (291.4). Required: C, 49.46; H, 4.51; N, 24.04; Found: C, 49.51; H, 4.32; N, 24.12.
5,6,7,8-Tetrahydrobenzo-2-(3-amino-4H,5H-5-pyrazolinon-1-yl)-3-methyl-thieno[2,3-d] pyrimidin-4-one (10b)
From 2b in 43% yield, m.p.292-4°C (dec.); IR (KBr) cm-1: 3219 (br, NH), 2927 (CH alkyl), 1688 (C=O), 1602 (C=N), 1521 (C=C) 1H-NMR (DMSO-d6) ppm: δ 1.92 (m, 4H, 2CH2), δ 2.82 (t, 2H, CH2), δ 3.16 (t, 2H, CH2), δ 3.23 (s, 2H, CH2), δ 3.76 (s, 3H, N-CH3), δ 10.31 (br, NH2, D2O ex-changeable); Analyses: C14H15N5O2S (317.4). Required: C, 52.97; H, 4.77; N, 22.07; Found: C, 53.07; H, 4.59; N, 22.19.
General procedure for the preparation of 11a,b
A mixture of compound 2a or 2b (10 mmol) with chloroacetone or phenacyl bromide (10 mmol) was heated under reflux 5 hr in dry xylene (30 mL). The solid precipitated that separated upon cooling was filtered off and crystallized from appropriate solvent to produce 11a,b in high yield .
1,5,7,8-Tetramethyl-5,6-dihydrothieno[2`,3`:6,5]pyrimido[2,1-c][1,2,4]triazin-6-one (11a)
From compound 2a (10 mmol) and chloroacetone (0.93g, 10 mmol). The compound was obtained as pale white crystals, crystallized from ethanol in 51% yield, m.p. 257-59°C (dec.); IR (KBr) cm-1: 3360 (brs, NH), 2950 (CH alkyl), 1689 (C=O), 1600 (C=N), 1550 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.23 (s,3H,CH3), δ 2.31 (s, 3H, CH3), δ 2.41 (s, 3H, CH3), δ 3.62 (s, 3H, N-CH3), δ 4.38 (brs, 1H, NH, D2O exchangeable), δ 9.02 (s,1H,triazine). Analyses: C12H14N4OS (262.4). Required: C, 54.93; H, 5.39; N, 21.36; Found: C, 55.11; H, 5.29; N, 21.07.
1-Phenyl-5,7,8-trimethyl-5,6-dihydrothieno[2`,3`:6,5]pyrimido[2,1-c][1,2,4] triazin-6-one (11b)
From compound 2a (10 mmol) and phenacylbromide (1.99g, 10 mmol). The compound was obtained as pale white crystals, crystallized from ethanol in 45% yield, m.p. 279-81°C (dec.); IR (KBr) cm-1: 3400 (brs, NH), 2958 (CH alkyl), 1694 (C=O), 1589 (C=N), 1559 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.24 (s, 3H, CH3), δ 2.35 (s, 3H, CH3), δ 3.74 (s, 3H, N-CH3), δ 7.02-7.84 (m, 5H, phenyl), δ 9.16 (s, 1H, triazine), δ 10.38 (brs,1H, NH, D2O exchangeable). Analyses: C17H16N4OS (324.4). Required: C, 62.94; H, 4.98; N, 17.28; Found: C, 62.78; H, 4.81; N, 16.86.
General procedure for the preparation of 12a-f
A mixture of compound 2a or 2b (10 mmol) and a β-diketone (10 mmol) in absolute ethanol (30 mL) was stirred under reflux for 5 hr. The reaction mixture was allowed to cool to 0°C for 3 hours. The precipitate was filtered off, dried and crystallized from the appropriate solvent to produce 12a-f in high yields.
3,5,6-Trimethyl-2-(3,5-dimethylpyrazolyl)-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (12a)
From compound 2a (10 mmol) and pentane-2,4-dione (1.00g, 10 mmol). The compound was ob-tained as pale light crystals, crystallized from dioxane in 83% yield, m.p. 217-19°C; IR (KBr) cm-1: 3060 (CH aryl), 2960 (CH alkyl), 1700 (C=O), 1600 (C=N), 1550 (C=C); 1H-NMR (DMSO-d6) ppm: δ 2.28 (s, 3H, CH3), δ 2.32 (s, 3H, CH3), δ 2.41 (s, 3H, CH3), δ 2.51 (s, 3H, CH3), δ 3.42 (s, 3H, N-CH3), δ 6.01 (s, 1H, pyrazole); 13C-NMR (DMSO-d6): 11.53, 1292, 13.16, 13.60 (4CH3), 31.69 (N-CH3), 107.53-151.42 (8C sp2 carbon atoms), 159.24 (C=O). Analyses: C14H16N4OS (288.4). Required: C, 58.31; H, 5.60; N, 19.43; Found: C, 57.97; H, 5.41; N, 19,26.
3,5,6-Trimethyl-2-(3,5-dimethyl-4-chloropyrazolyl)-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (12b)
From compound 2a (10 mmol) and 3-chloropentane-2,4-dione (1.34g, 10 mmol). The compound was obtained as light white crystals, crystallized from DMF in 92% yield, m.p. 259-61°C (dec.); IR (KBr) cm-1: 2960 (CH alkyl), 1680 (C=O), 1600 (C=N), 1580 (C=C) ; 1H-NMR (CDCl3) ppm: δ 2.29 (s, 3H, CH3), δ 2.34 (s, 3H, CH3), δ 2.41 (s, 3H, CH3), δ 2.51 (s, 3H, CH3), δ 3.44 (s, 3H, N-CH3); 13C-NMR (CDCl3): 10.23, 11.56, 12.91, 13.19 (4CH3), 31.81 (N-CH3), 111.26-148.67 (7C sp2 carbon atoms), 158.96 (C-Cl), 159.13 (C=O). Analyses: C14H15ClN4OS (322.8). Required: C, 52.15; H, 4.69; N, 17.36; Found: C, 51.89; H, 4.45; N, 17.25.
3,5,6-Trimethyl-2-(3-methyl-5-trifluromethylpyrazolyl)-3,4-dihydrothieno[2,3-d]pyrimidin-4-one (12c)
From compound 2a (10 mmol) and 1,1,1-trifluro-2,4-pentanedione (1.54g, 10 mmol). The com-pound was obtained as a pale light colorless crystals, crystallized from ethanol in 82% yield, m.p. 231-33°C; IR (KBr) cm-1: 2980 (CH alkyl), 1680 (C=O), 1600 (C=N), 1560 (C=C); 1H-NMR (CDCl3:DMSO-d6/4:1) ppm: δ 2.10 (s, 3H, CH3), δ 2.35 (s, 3H, CH3), δ 2.451 (s, 3H, CH3), δ 2.56 (s, 3H, N-CH3), δ 8.32 (s, 1H, pyrazole); 13C-NMR (CDCl3:DMSO-d6/4:1): 11.53, 1292, 13.16, 13.60 (4CH3), 31.69 (N-CH3), 107.53-151.42 (8C sp2 carbon atoms), 159.24 (C=O). Analyses: C14H13F3N4OS (342.4). Required: C, 49.11; H, 3.83; N, 16.37; Found: C, 49.02; H, 3.71; N, 16.18.
3-Methyl-5,6,7,8-tetrahydrobenzo-2-(3,5-dimethylpyrazolyl)3,4-dihydrothieno[2,3-d]pyrimidin-4-one (12d)
From compound 2b (10 mmol) and pentane-2,4-dione (1.00g, 10 mmol). The compound was ob-tained as pale yellow crystals, crystallized from dioxane/ethanol in 71% yield, m.p. 248-50°C (dec.); IR (KBr) cm-1: 3060 (CH aryl), 2960 (CH alkyl), 1700 (C=O), 1600 (C=N), 1550 (C=C); 1H-NMR (DMSO-d6) ppm: δ 1.87 (m, 4H, 2CH2), δ 2.25 (s, 3H, CH3), δ 2.28 (s, 3H, CH3), δ 2.76 (m, 2H, CH2), δ 3.07 (m, 2H, CH2), δ 3.41 (s, 3H, N-CH3) and δ 6.06 (s, 1H, pyrazole). Analyses: C16H18N4OS (314.4). Required: C, 61.12; H, 5.78; N, 17.82; Found: C, 61.09; H, 5.63; N, 17,56.
3-Methyl-5,6,7,8-tetrahydrobenzo-2-(3,5-dimethyl-4-chloropyrazolyl)-3,4-dihydrothieno[2,3-d]pyri-midin-4-one (12e)
From compound 2b (10 mmol) and 3-chloropentane-2,4-dione (1.34g, 10 mmol). The compound was obtained as yellow crystals, crystallized from dimethylformamide in 81% yield, m.p. 278-80°C (dec.); IR (KBr) cm-1: 2960 (CH alkyl), 1689 (C=O), 1608 (C=N), 1580 (C=C); 1H-NMR (CDCl3) ppm: δ 1.88 (m, 4H, 2CH2), δ 2.29 (s, 3H, CH3), δ 2.33 (s, 3H, CH3), δ 2.79 (m, 2H, CH2), δ 3.04 (m, 2H, CH2) and δ 3.44 (s, 3H, N-CH3). Analyses: C16H17ClN4OS (348.9). Required: C, 55.09; H, 4.92; N, 16.06; Found: C, 55.18; H, 4.79; N, 15.98.
3-Methyl-5,6,7,8-tetrahydrobenzo-2-(3-methyl-5-trifluromethylpyrazolyl)-thieno[2,3-d]pyrimidin-4-one (12f)
From compound 2b (10 mmol) and 1,1,1-trifluro-2,4-pentanedione (1.54g, 10 mmol). The com-pound was obtained as pale white crystals, crystallized from ethanol in 76% yield, m.p. 293-95°C (dec.); IR (KBr) cm-1: 2980 (CH alkyl), 1680 (C=O), 1600 (C=N), 1560 (C=C); 1H-NMR (CDCl3:DMSO-d6/4:1) ppm: δ 1.85 (m, 4H, 2CH2), δ 2.11 (s, 3H, CH3), δ 2.72 (m, 2H, CH2), δ 2.99 (m, 2H, CH2), δ 3.56 (s, 3H, N-CH3) and δ 8.32 (s,1H, pyrazole). Analyses: C16H15F3N4OS (368.4). Required: C, 52.17; H, 4.11; N, 15.21; Found: C, 52.01; H, 3.99; N, 15.03.