Synthesis and Antifungal Activity of Musa Phytoalexins and Structural Analogs
Abstract
:Introduction
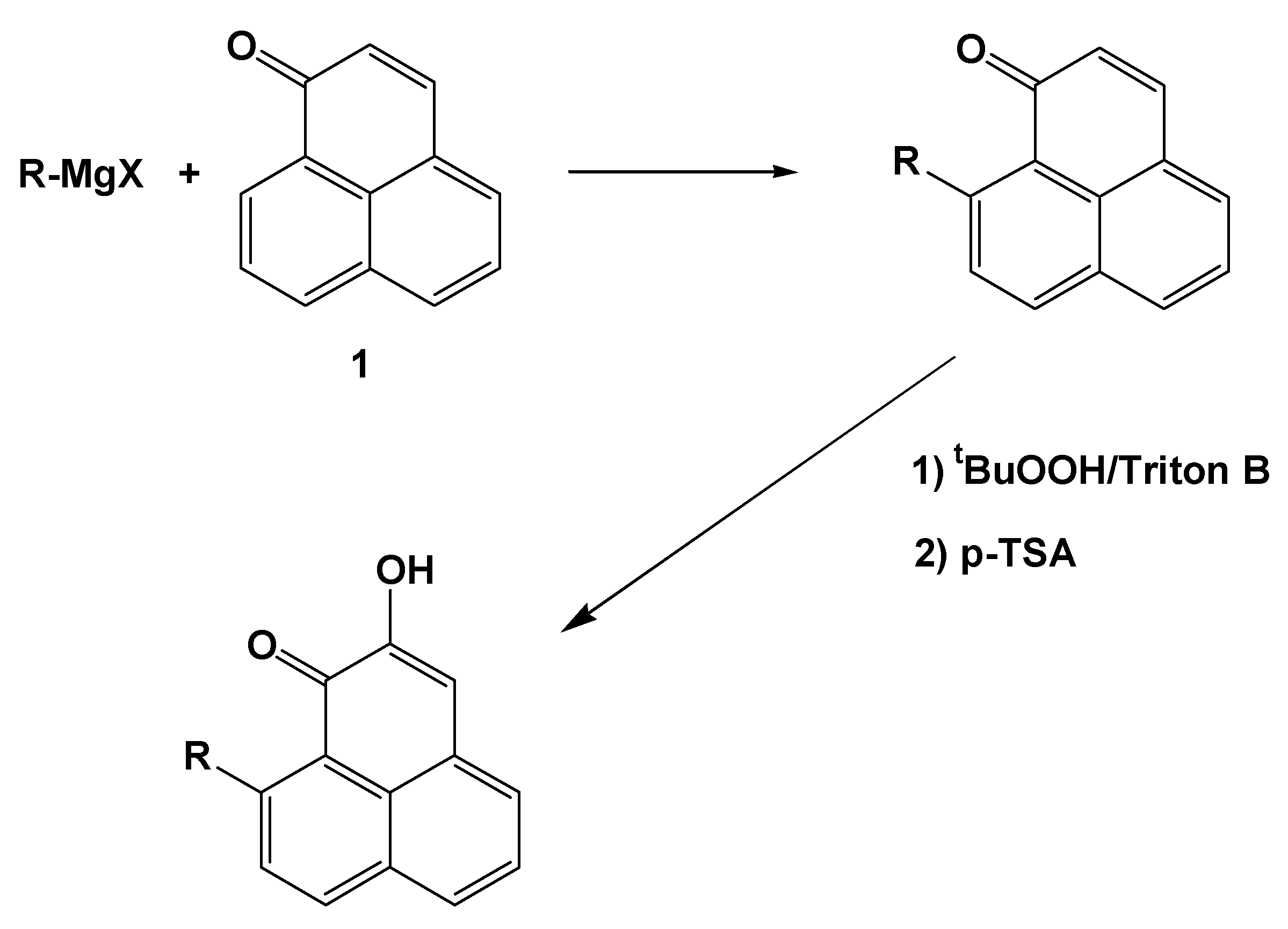
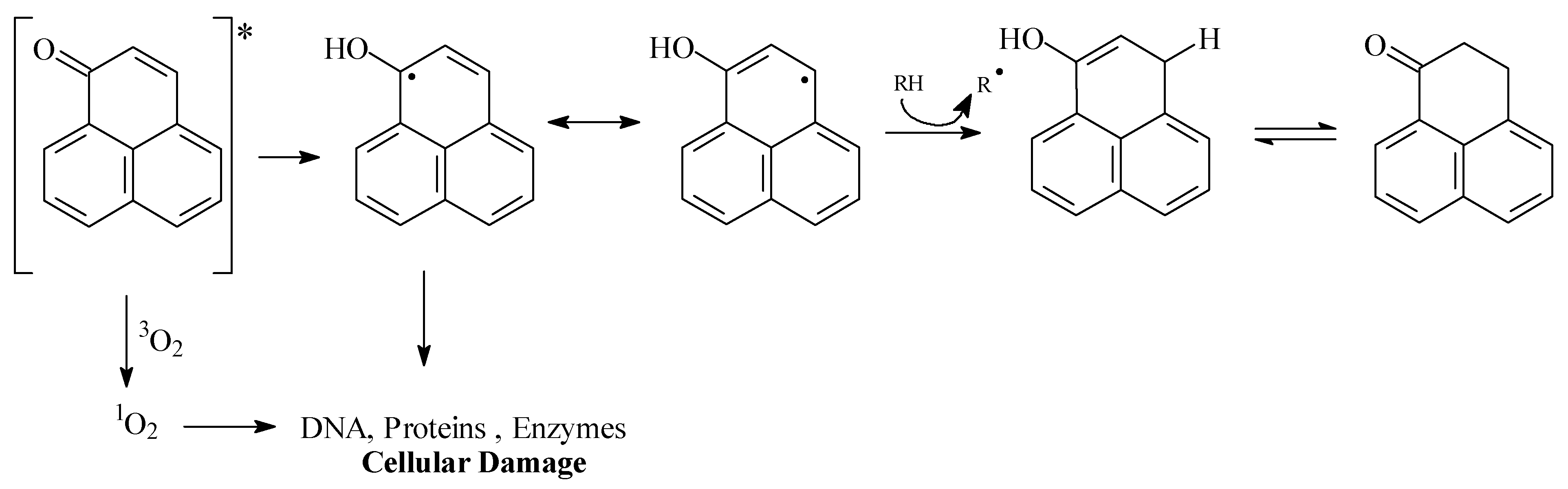
Results and Discussion
Experimental
General
General synthetic procedures
Spectral data
Perinaphthenone (1)
9-Phenylperinaphthenone (2)
9-(p-Methoxyphenyl)perinaphthenone (3)
9-(2’,4’-Dimethoxyphenyl)-perinaphthenone (4)
9-(3’,4’-Methylenedioxy)perinaphtenone (5)
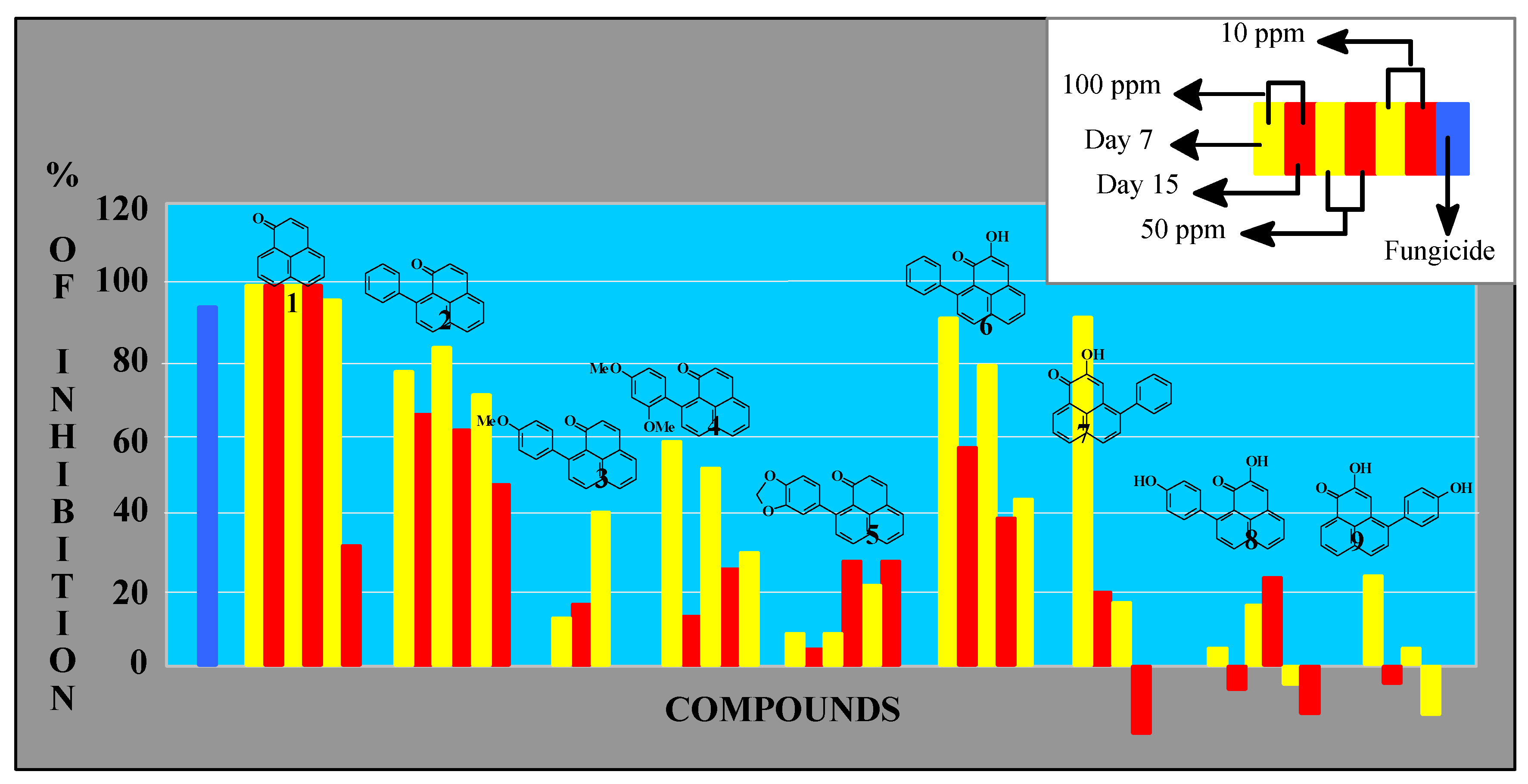
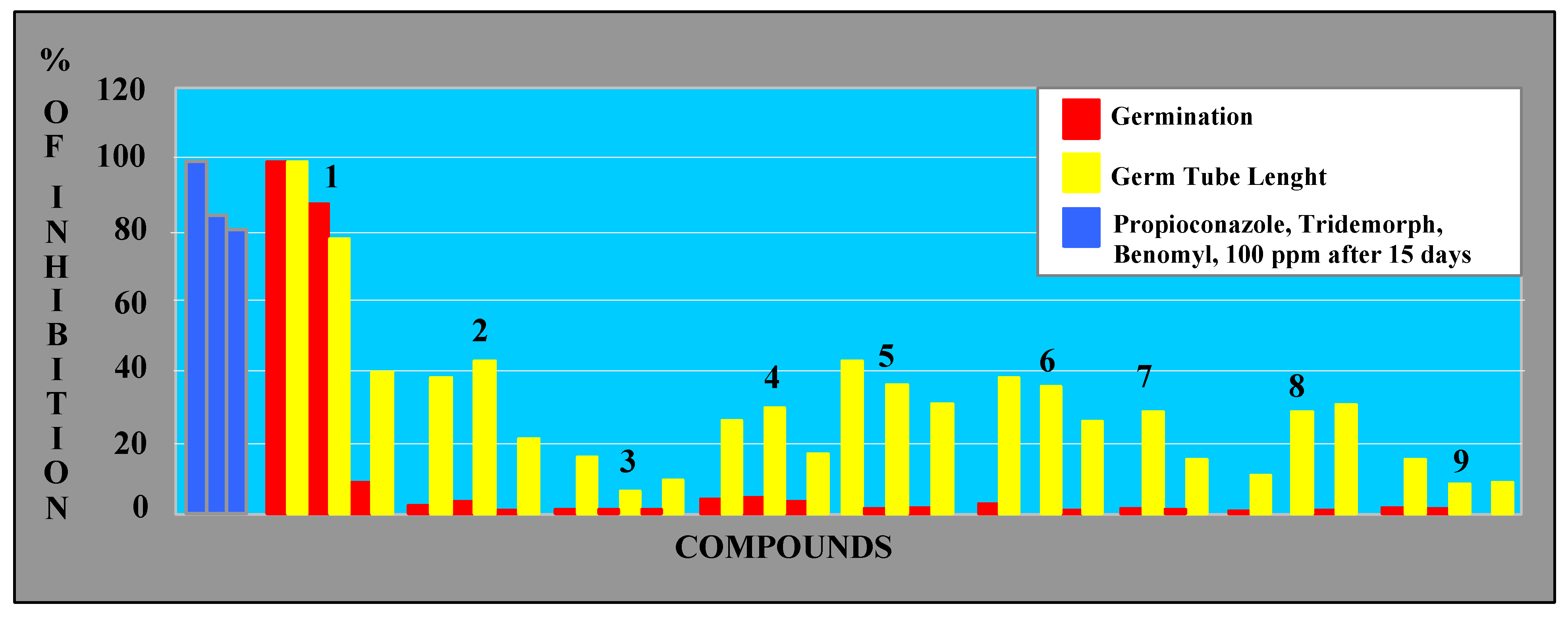
Antifungal activity
Inhibition of mycelial growth [10]
Inhibition of spore germination
Acknowledgements
References and Notes
- Keen, N. Evaluation of Role of Phytoalexins. In Plant Disease Control; Stapless, R., Toenniessen, G., Eds.; John Wiley: New York, 1981; p. 339. [Google Scholar]
- Simmonds, N. W. Bananas, 2 nd ed.; Longman: London, 1982; p. 512. [Google Scholar]
- Luis, J.G.; Quiñones, A.; Echeverri, F.; Kishi, P.; Garcia, F. Musanolones: four 9-phenylphenalenones from rhizomes of M. acuminata. Phytochemistry 1996, 41, 753–757. [Google Scholar] [CrossRef]
- Luis, J.G.; Echeverri, F.; Quiñones, W.; Brito, I.; Lopez, M.; Torres, F.; Cardona, G.; Aguiar, Z.; Rojas, M. Irenolone and emenolone: two new types of phytoalexin from Musa paradisiaca. J. Org. Chemistry 1993, 58, 4306–4308. [Google Scholar] [CrossRef]
- Luis, J.G.; Quiñones, W.; Echeverri, F.; Grillo, T.; Perales, A.; Gonzalez, J.A. Intermediates with biosynthetic implications in de novo production of phenylphenalenone-type phytoalexins by Musa acuminata. Revised structure of emenolone. Tetrahedron 1995, 51, 4117–30. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Li, J.; Xie, L. [3+3] benzannulations of benzenoid- and heteroaromatic-ring systems. Tetrahedron 1999, 55, 8263–8293. [Google Scholar] [CrossRef]
- Oliveros, E.; Bossmann, S.; Nonell, S.; Marti, C.; Heit, G.; Tröscher, G.; Neuner, A.; Martínez, C.; Braun, A. Further characterization of perinaphthenone (1H-phenalen-1-one) as O2 (1Δg) standard: photochemical decomposition in 1,4-dioxane and DMA. http://www.photobiology.com/iupac98/ nonel/pn_oliv.htm.
- Park, J.; English, D. S.; Wannamuelher, Y.; Carpenter, S.; Petrich, J. W. The role of oxygen in the antiviral activity of hypericin and hypocrellin. Photochemistry & Photobiology 1998, 68, 593–597. [Google Scholar]
- Kamo, T.; Kato, N.; Hirai, N.; Tsuda, M.; Fujioka, D.; Ohigashi, H. Phenylphenalenone-type phytoalexins from unripe bungulan banana fruit. Bios. Biotech. & Biochemistry 1998, 62, 95–101. [Google Scholar]
- Bailey, J.; Carter, A.; Skipp, A. The use and interpretation of bioassay for fungitoxicity of phytoalexins in agar media. Physiol. Plant Pathol. 1976, 8, 189–194. [Google Scholar] [CrossRef]
- Molina, G.; Krausz, J. A phytotoxic activity in extracts of broth cultures of Mycosphaerella fijiensis var. difformis and its use to evaluate host resistance to Black Sigatoka. Plant Disease 1989, 73, 142–143. [Google Scholar]
- Sample Availability: Available from MDPI.

© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Quiñones, W.; Escobar, G.; Echeverri, F.; Torres, F.; Rosero, Y.; Arango, V.; Cardona, G.; Gallego, A. Synthesis and Antifungal Activity of Musa Phytoalexins and Structural Analogs. Molecules 2000, 5, 974-980. https://doi.org/10.3390/50700974
Quiñones W, Escobar G, Echeverri F, Torres F, Rosero Y, Arango V, Cardona G, Gallego A. Synthesis and Antifungal Activity of Musa Phytoalexins and Structural Analogs. Molecules. 2000; 5(7):974-980. https://doi.org/10.3390/50700974
Chicago/Turabian StyleQuiñones, Winston, Gustavo Escobar, Fernando Echeverri, Fernando Torres, Yoni Rosero, Victor Arango, Gloria Cardona, and Adriana Gallego. 2000. "Synthesis and Antifungal Activity of Musa Phytoalexins and Structural Analogs" Molecules 5, no. 7: 974-980. https://doi.org/10.3390/50700974




