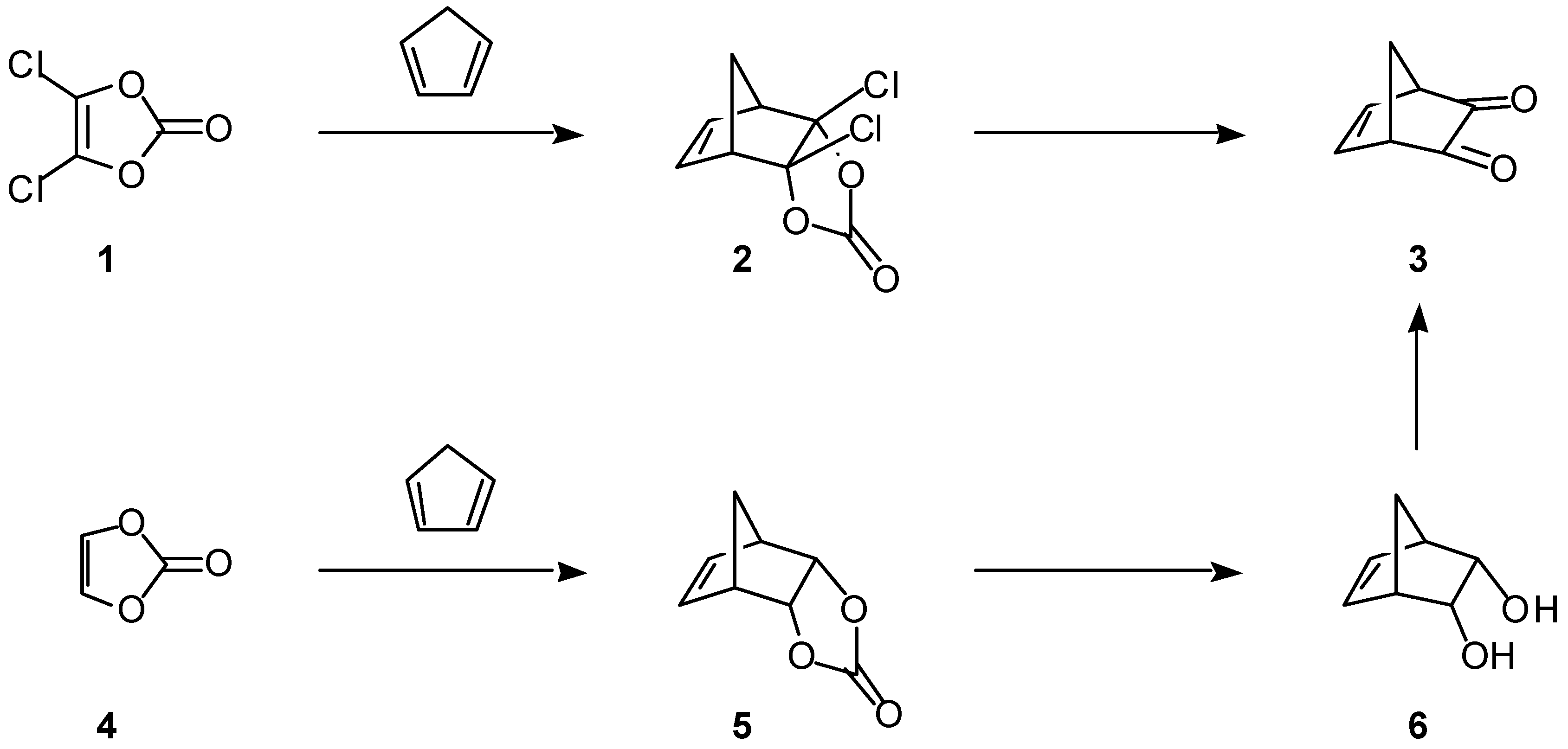
Bicyclo[2.2.1]hept-5-ene-2,3-dione (3)
Trifluoroacetic anhydride (35.92 g, 171 mmol) was added dropwise over 40 min to a solution of dimethyl sulfoxide (14.79 g, 189 mmol) in dichloromethane (100 mL) cooled with a dry ice-acetone bath. To this solu-tion was added a solution of bicyclo[2.2.1]hept-5-ene-2-
endo,3-
endo-diol (
6) (7.50 g, 60 mmol) in dichlo- romethane (30 mL) over 10 min. The mixture was further stirred at -78°C for 2 h. Triethylamine (31.98 g, 316 mmol) was added to the solution. The mixture was stirred for 3 h and allowed to warm up to room tem-perature. 2M Hydrochloric acid was added and the product was extracted with dichloromethane. The organic phase was dried with sodium sulfate. After removal of the solvent, the resulting yellow oil was distilled under vacuum to give
3 (5.31 g, 73%) as a yellow oil which solidified after cooling: bp 129°C (10 Torr); mp 40°C (lit [
3] mp 43°C) ;
1H NMR (CDCl
3) δ=2.49 (1H, d,
J=11, 7-H), 3.00 (1H, dt,
J=11 and 2, 7-H), 3.32 (2H, m, 1-H and 4-H), 6.51 (2H, br s, 5-H and 6-H);
13C NMR (CDCl
3) δ=43.6 (C-7), 51.4 (C-1 and C-4), 137.7 (C-5 and C-6), 195.6 (C-2 and C-3); IR (KBr) 1765 cm
-1; MS m/z 122 (9; M
+), 95 (16; M - C
2H
3), 66 (100, C
5H
6).
A similar reaction of 6 (0.65 g, 5.8 mmol) with dimethyl sulfoxide (1.19 g, 15 mmol) and oxalyl chloride (1.65 g, 13 mmol), followed by the addition of triethylamine (2.70 g, 27 mmol) provided 3 (0.43 g, 61%).
5,6,7,8-Tetrahydro-5,8-methanoquinoxaline-6-exo,-7-exo-diol (10)
To a solution of osmium tetroxide (1 mg, 0.004 mmol) and N-methylmorpholine N-oxide (246 mg, 2.1 mmol) in a mixture of acetone ( 5 mL) and water (10 mL) was added the norbornadiene-fused pyrazine 7 (100 mg, 0.7 mmol) and the mixture was stirred at room temperature for 68 h. Sodium sulfite (3 mg, 0.03 mmol) and florisil (0.5 g) was added to the reaction mixture and insoluble materials were filtered through celite. Aqueous sodium hydroxide solution was added to the filtrate and products were extracted with dichlo- romethane (6 ° 20 mL). The combined organic phases were dried over sodium sulfate. After removal of the solvent, the residue was purified by column chromatography (silica gel, ethyl acetate), and the resulting solid was recrystallized from chloroform to give 10 (112 mg, 90%) as a white solid: mp 186-187°C; 1H NMR (CDCl3) δ=2.17 (1H, dm, J=14, 9-Hs), 3.00 (1H, dt, J=14 and 2, 9-Ha), 3.45 (2H, t, J=2, 5-H and 8-H), 3.99 (2H, br s, 6-H and 7-H), 4.15 (2H, s, OH, disappeared by D2O addition), 8.16 (2H, s, 2-H and 3-H); 13C NMR (CDCl3) δ=41.2 (C-5 and C-8), 51.4 (C-9), 69.7 (C-2 and C-3), 141.7 (C-6 and C-7), 161.1 (C-4a and C-8a); IR (KBr) 3490, 3097, 1365, 1160 cm-1; MS m/z 178 (33; M+), 149 (31; M - CHO), 119 (100, M - 2CHO - H). Anal. Found: C, 60.52; H, 5.96; N, 15.67%. Calcd for C9H10N2O2: C, 60.66; H, 5.66; N, 15.72%.
1,2,3,4-Tetrahydro-1,4-methanophenazine-2-exo,3-exo-diol (11)
By a similar procedure as described for 10, the norbornadiene-fused quinoxaline 8 (349 mg, 1.8 mmol) provided 11 (366 mg, 89%) as a white solid after recrystallization from chloroform: mp 183-184°C; 1H NMR (CDCl3) δ=2.23 (1H, dm, J=11, 11-Hs), 2.65 (1H, d, J=11, 11-Ha), 3.58 (2H, s, 1-H and 4-H), 3.77 (2H, s, OH, disappeared by D2O addition), 4.14 (2H, s, 2-H and 3-H), 7.70 (2H, m, 7-H and 8-H), 8.00 (2H, m, 6-H and 9-H); 13C NMR (CDCl3) δ=39.0 (C-1 and C-4), 51.3 (C-11), 76.3 (C-2 and C-3), 128.7 (C-7 and C-8), 129.4 (C-6 and C-9), 141.4 (C-5a and C-9a), 160.8 (C-4a and C-10a); IR (KBr) 3430, 3074, 1076 cm-1; MS m/z 228 (59; M+), 199 (31; M - CHO), 169 (100, M - 2CHO - H). Anal. Found: C, 68.41; H, 5.30; N, 12.27%. Calcd for C13H12N2O2: C, 68.68, H, 5.21; N, 12.01%.
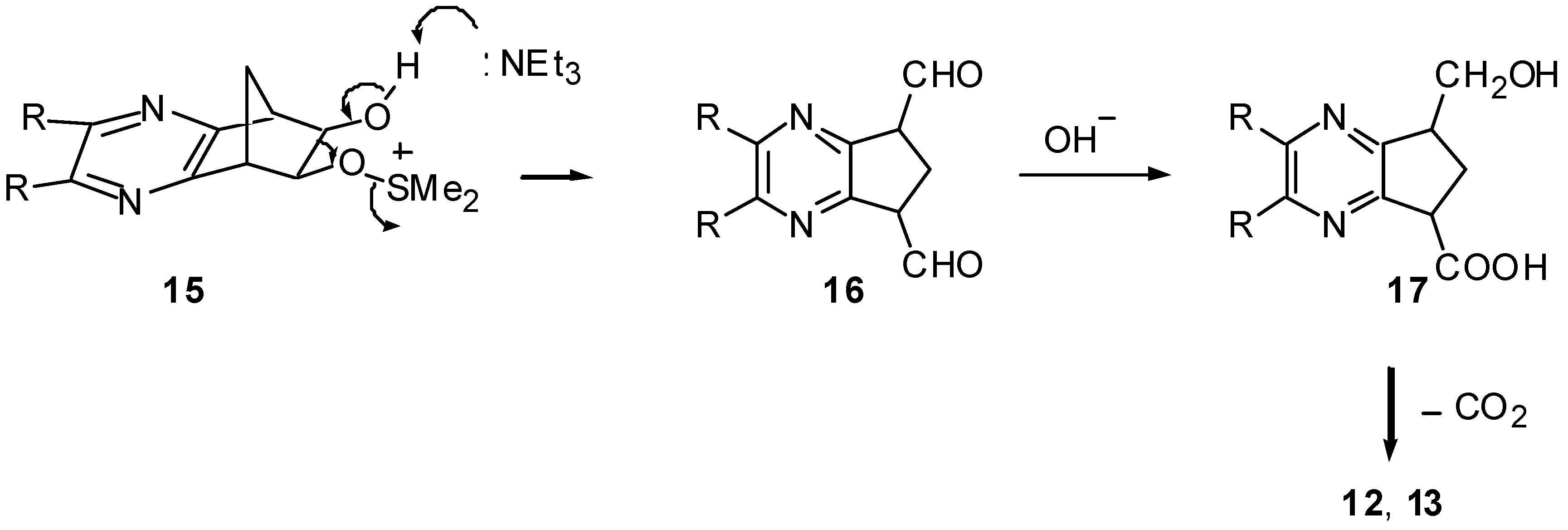
The Swern Oxidation of 10
To a cooled (-78°C) solution of dimethyl sulfoxide (125 mg, 1.6 mmol) in dichloromethane (10 mL) was added trifluoroacetic anhydride (315 mg, 1.5 mmol) over 5 min. A solution of 10 (89 mg, 0.5 mmol) in a 1:1 mixture (5 mL) of dichloromethane and dimethyl sulfoxide was added over 10 min and the mixture was stirred at -78°C for 3 h. Triethylamine (268 mg, 2.7 mmol) was introduced to the solution. The mixture was stirred at -78°C for 1.5 h and allowed to warm up to room temperature. Aqueous sodium hydroxide solution was added and the product was extracted with dichloromethane (6 ° 20 mL). The combined organic phase was washed with water and dried over sodium sulfate. After removal of the solvent, the residue was separated by column chromatography (silica gel, ethyl acetate) to give (6,7-dihydro-5H-cyclopentapyrazin-5-yl)methanol (12) (56 mg, 75%) as a colorless liquid: 1H NMR (CDCl3) δ=1.98 (1H, ddd, J=19, 11 and 9, 6-H), 2.37 (1H, ddd, J=19, 8 and 6, 6-H), 3.07 (2H, dd, J=9 and 6, 7-H), 3.43 (1H, m, 5-H), 3.80-4.01 (3H, m, CH2OH and OH), 8.24 (1H, br s, 2-H or 3-H), 8.30 (1H, br s, 3-H or 2-H); 13C NMR (CDCl3) δ=24.2 (C-6), 30.8 (C-7), 45.1 (C-5), 64.9 (CH2OH), 141.6 (C-2 or C-3), 142.7 (C-3 or C-2), 159.9, 160.2; IR (KBr) 3347, 2946, 1388, 1160 cm-1; MS m/z 150 (2; M+), 119 (100, M - CH2OH). HR-MS (FAB+) found: 151.0899 (M + 1). Calcd for C8H10N2O: 151.0872 (M + 1).
The Swern Oxidation of 11
By a similar procedure as described for the oxidation of 10, the norbornadiene-fused quinoxaline 11 (114 mg, 0.5 mmol) provided (2,3-dihydro-1H-cyclopenta[b]quinolin-1-yl)methanol (13) (58 mg, 63%) as a white solid after recrystallization from hexane: mp 83-84°C; 1H NMR (CDCl3) δ=1.97 (1H, ddd, J=18, 13, and 9, 2-H), 2.47 (1H, ddd, J=18, 8, and 5, 2-H), 3.02 (1H, s, OH), 3.22 (2H, dd, J=9 and 5, 3-H), 3.58 (1H, m, 1-H), 3.98 (1H, dd, J=11 and 8, CH2OH), 4.09 (1H, dd, J=11 and 5, CH2OH), 7.62 (2H, m), 8.02 (2H, m); 13C NMR (CDCl3) δ=24.3 (C-2), 31.3 (C-3), 45.0 (C-1), 65.1 (CH2OH), 128.7, 128.8, 128.9, 129.1, 141.0, 141.9, 160.6, 161.8; IR (KBr) 3241, 2964, 1342 cm-1; MS m/z 200 (5; M+), 182 (16, M M - H2O), 169 (100, M - CH2OH). HR-MS (FAB+) found: 201.1055 (M + 1). Calcd for C12H12N2O: 201.1028 (M + 1).
A similar Swern oxidation of 11 (68 mg, 0.3 mmol) by the use of oxalyl chloride (114 mg, 0.9 mmol) in-stead of trifluoroacetic anhydride also afforded 13 (38 mg, 63%).