Introduction
Oroidin (
1) is a major metabolite of several species of marine sponges of the genus
Agelas and is the basis of the oroidin group of alkaloids. It was first isolated in 1971 from
Agelas oroides [
1] but its structure misassigned. A revised structure was established in 1973 [
2] and proven by total synthesis in 1986 [
3]. Subsequently, many other species of
Agelas, Axinella, Acanthella, Hymeniacidon, Phakellia and
Pseudaxinyssa have been reported to contain high levels of oroidin or cyclic analogues. It is thus clear that oroidin alkaloids are useful chemotaxonomic markers for axinellid sponges that were once allied with the Agelasida.
In 1981, Faulkner and co-workers [
4] isolated sceptrin (
2) from
Agelas sceptrum. Formally, sceptrin is related to debromooroidin by a head-to-head [2 + 2]-cycloaddition that must be photochemically allowed. As oroidin is achiral, if this were a photochemical reaction, sceptrin ought to have been isolated as a racemic mixture. In contrast, sceptrin is chiral ([α]
D - 7.4°) suggesting that it is formed by an enzyme catalysed reaction. If this is the case, it will be the first example of a biological [2 + 2] cycloaddition or, indeed, any pericyclic reaction. Slight variations on the structure of sceptrin (eg dibromosceptrin, debromosceptrin, oxysceptrin (
3) and nakamuric acid (
4)) have been isolated in subsequent work from
Agelas cf
nemoechinata [
5],
A. conifer [
6] and
A. nakamurai [
7].
These compounds are also of pharmaceutical interest as many have shown α-adrenoceptor blocking activity that does not interfere with the action of potassium chloride or seratonin [
8]. Specifically, sceptrin (
2), and its analogues have potent antibacterial/antifungal activities [
9], anti-muscarinic activity [
10], anti-histaminic activity [
11] and oxysceptrin is also a potent actomyosin ATPase activator [
5]. As a first step toward isolation of enzymes involved in the biosynthesis of sceptrin, we need to find a local sponge that contains this compound.
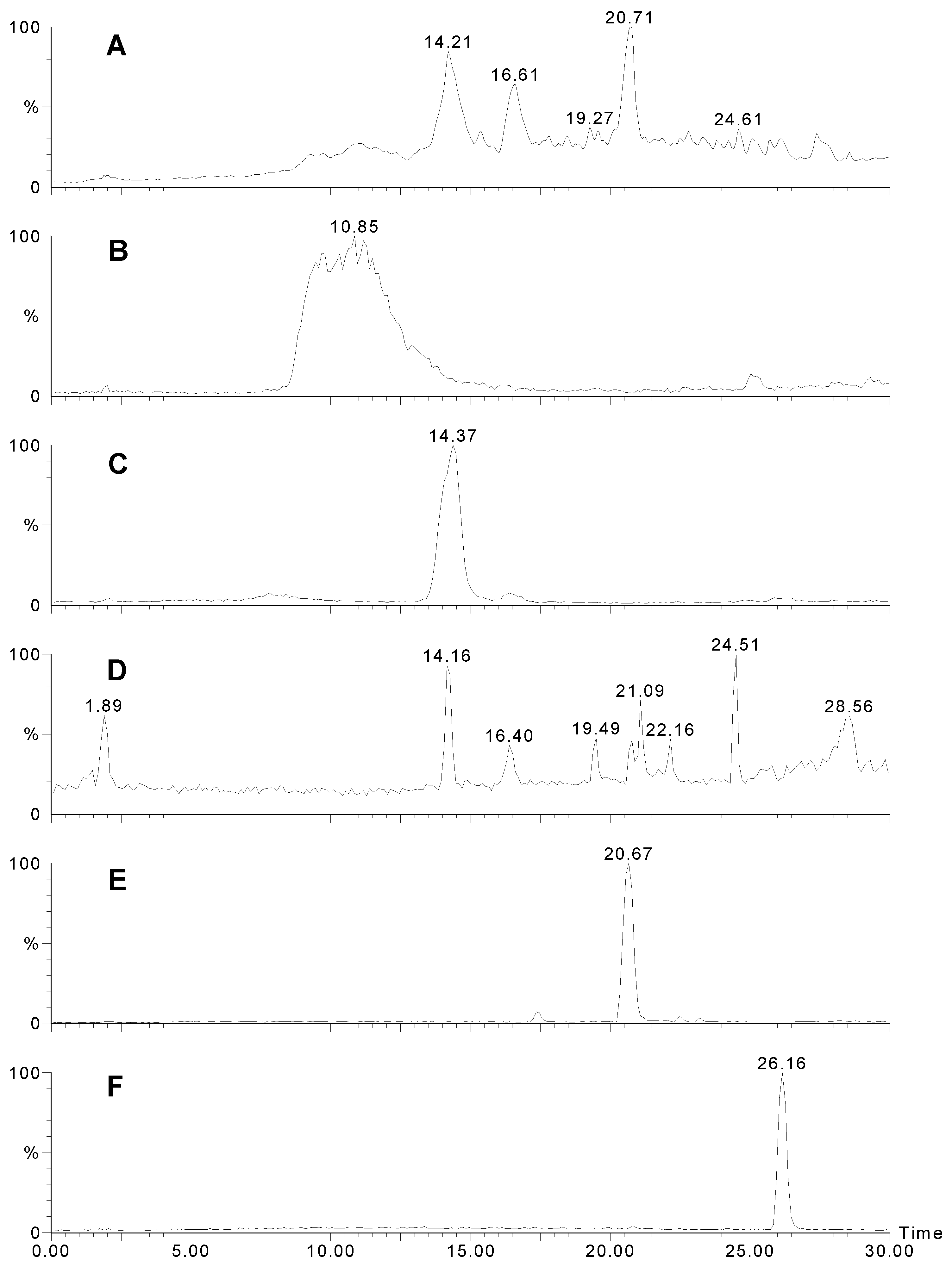
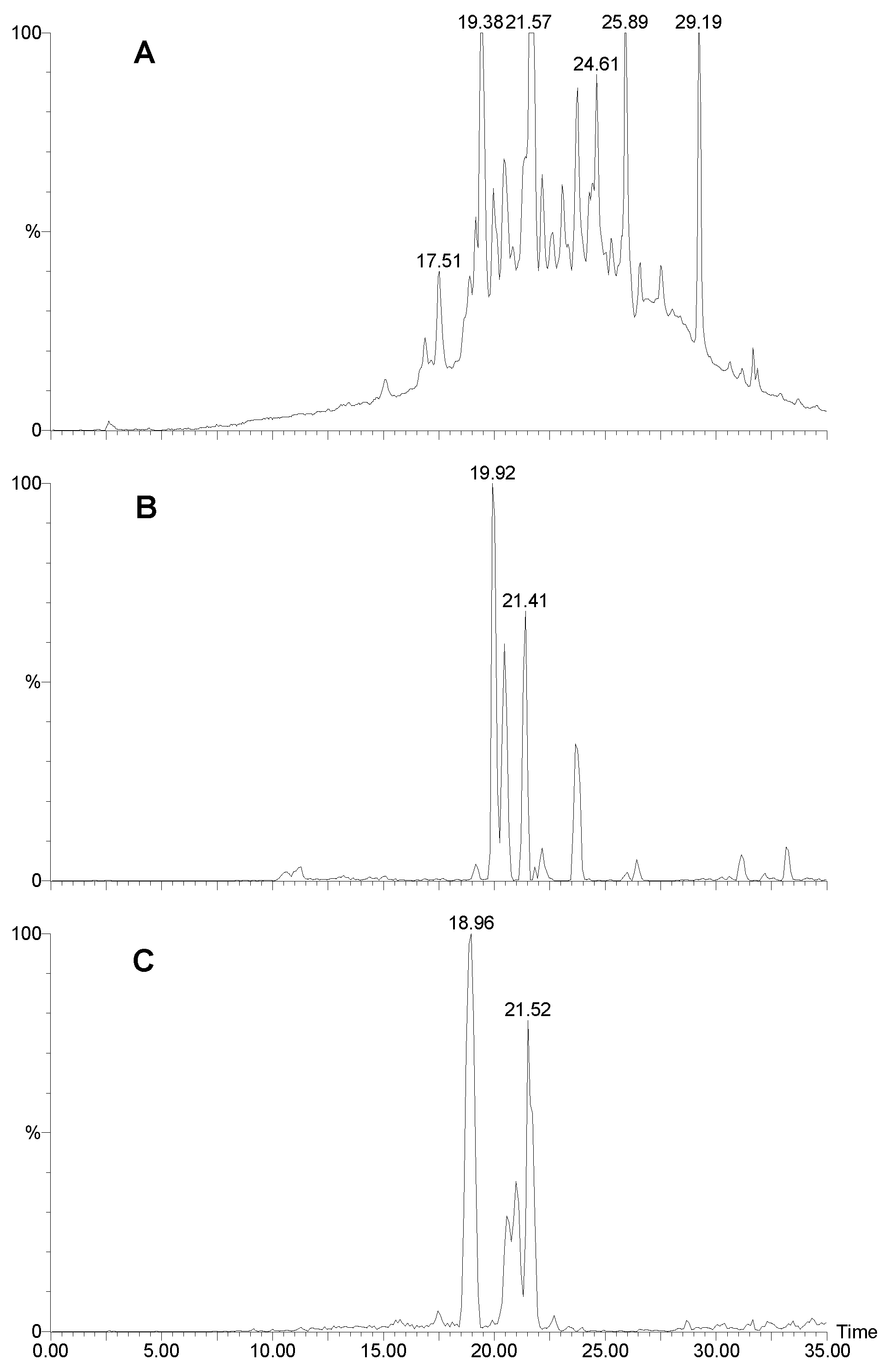
Figure 1.
LCMS traces for Agelas sp. 1A. A) UV trace at 280 nm. B) Positive ion mass chromatogram of m/z 621. Sceptrin elutes as a broad peak due to the two very basic guanidinium groups. C) Positive ion mass chromatogram of m/z 637. D) Positive ion mass chromatogram of m/z 189+191. Many other bromopyrroles also contain these peaks as a fragment. E) Positive ion mass chromatogram of m/z 584. F) Negative ion mass chromatogram of m/z 268.
Figure 1.
LCMS traces for Agelas sp. 1A. A) UV trace at 280 nm. B) Positive ion mass chromatogram of m/z 621. Sceptrin elutes as a broad peak due to the two very basic guanidinium groups. C) Positive ion mass chromatogram of m/z 637. D) Positive ion mass chromatogram of m/z 189+191. Many other bromopyrroles also contain these peaks as a fragment. E) Positive ion mass chromatogram of m/z 584. F) Negative ion mass chromatogram of m/z 268.
Results and Discussion
Four specimens of
Agelas spp were collected in Western Australia, extracted, and the crude extracts, (after treatment) were subject to analysis by analytical HPLC and LC-MS. The results are summarised in
Table 1 and
Table 2. Only
Agelas sp. 1A (WAM Z1194) showed evidence of sceptrin.
Figure 1A shows the HPLC trace (UV; 280 nm) of the extract, sceptrin eluting as a very broad peak (
Fig 1B) with a molecular ion at
m/z 619/621/623. A doubly charged ion is evident at
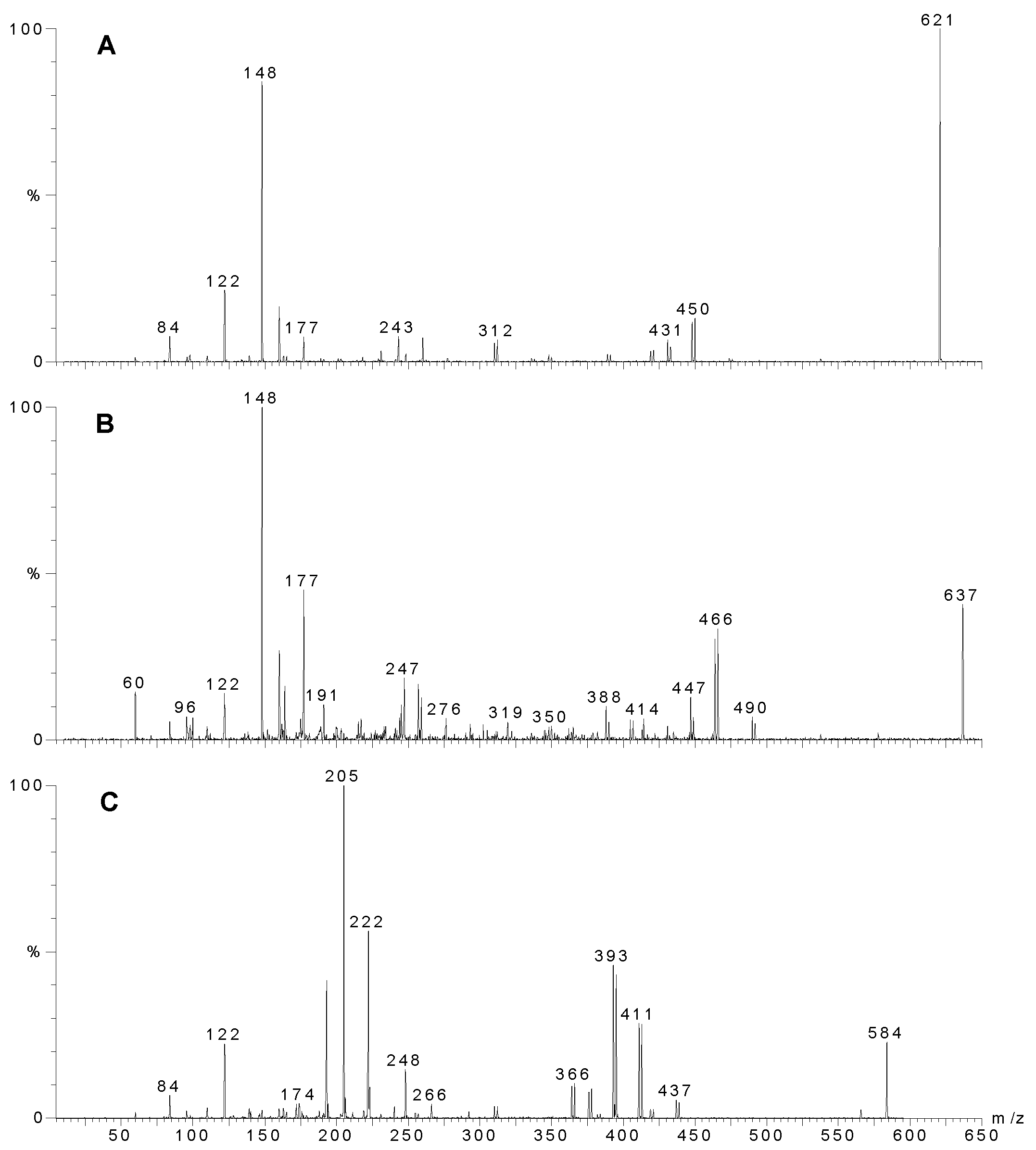
m/z 311. The MS-MS of the 621 peak is shown in
Fig. 2A. Note the loss of 172, characteristic of the bromo-2-pyrrolecarbonyl fragment. Subsequent loss of ammonia yields a fragment at
m/z 431/433. The next major peak corresponds to oxysceptrin (
3) with a retention time of 14.4 min. (
Fig. 1C) and a molecular ion at
m/z 635/637/639. The major fragmentations of
3 (
Fig 2B) showed loss of 172 then 17 like sceptrin. Peaks for 4-bromo-2-pyrrolecarboxamide (14.2;
Figure 1D), nakamuric acid (
4) (20.7;
Figure 1E) and 4,5-dibromo-2-pyrrolecarboxylic acid (26.2 min.;
Figure 1F) followed, the latter characteristically giving a strong negative ion spectrum. Nakamuric acid (20.7 min.) was characterised by a molecular ion at
m/z 582/584/586. MS-MS of the 584 amu peak (
Fig. 2C) again showed loss of 172 (as for sceptrin and oxysceptrin) but subsequent loss of water (18) instead of ammonia (17) was observed.
Figure 2.
MS-MS of individual compounds from Agelas sp. 1A. A) Sceptrin, precursor ion m/z 621. B) Oxysceptrin, precursor ion m/z 637, C) Nakamuric acid, precursor ion m/z 584. The most intense ion of the molecular ion cluster was used for MS-MS analysis as this ion contained both the 79 and 81 isotopes of bromine and hence fragments that still contained a bromine atom could be readily identified.
Figure 2.
MS-MS of individual compounds from Agelas sp. 1A. A) Sceptrin, precursor ion m/z 621. B) Oxysceptrin, precursor ion m/z 637, C) Nakamuric acid, precursor ion m/z 584. The most intense ion of the molecular ion cluster was used for MS-MS analysis as this ion contained both the 79 and 81 isotopes of bromine and hence fragments that still contained a bromine atom could be readily identified.
Table 1.
Major peaks from LC-MS from Agelas sp. 1 (A and B) with tentative structural assignments.
Table 1.
Major peaks from LC-MS from Agelas sp. 1 (A and B) with tentative structural assignments.
| | Agelas sp. 1A | | |
| RT | Molecular ion | MW | Tentative structure |
| 10.8 | 619/621/623 | 618 | sceptrin (2) |
| 14.2 | 189/191 | 188 | 4-bromopyrrole-2-carboxamide |
| 14.4 | 635/637/639 | 634 | oxysceptrin (3) |
| 15.4 | 687/689/691 | 686 | oxysceptrin acetate |
| 16.6 | 679/681/683 | 678 | |
| 17.8 | 610/612/614 | 609 | |
| 18.5 | 642/644/646 | 641 | |
| 19.3 | 596/598/600 | 595 | |
| 20.7 | 582/584/586 | 581 | nakamuric acid (4) |
| 26.2 | 266/268/270* | 267 | 4,5-dibromopyrrole 2-carboxylic acid |
| | Agelas sp. 1B | | |
| 14.5 | 189/191 | 188 | 4 (or 5?)-bromopyrrole-2-carboxamide |
| 17.9 | 635/637/639 | 634 | oxysceptrin (3) |
| 19.2 | 619/621/623 | 618 | sceptrin (2) |
| 19.2 | 687/689/691 | 678 | oxysceptrin acetate |
| 19.9 | 357/359* | 358 | |
| 21.1 | 265/267/269* | 266 | 4,5-dibromopyrrole 2-carboxamide |
| 22.5 | 582/584/586 | 581 | nakamuric acid (4) |
| 24.9 | 266/268/270* | 267 | 4,5-dibromopyrrole 2-carboxylic acid |
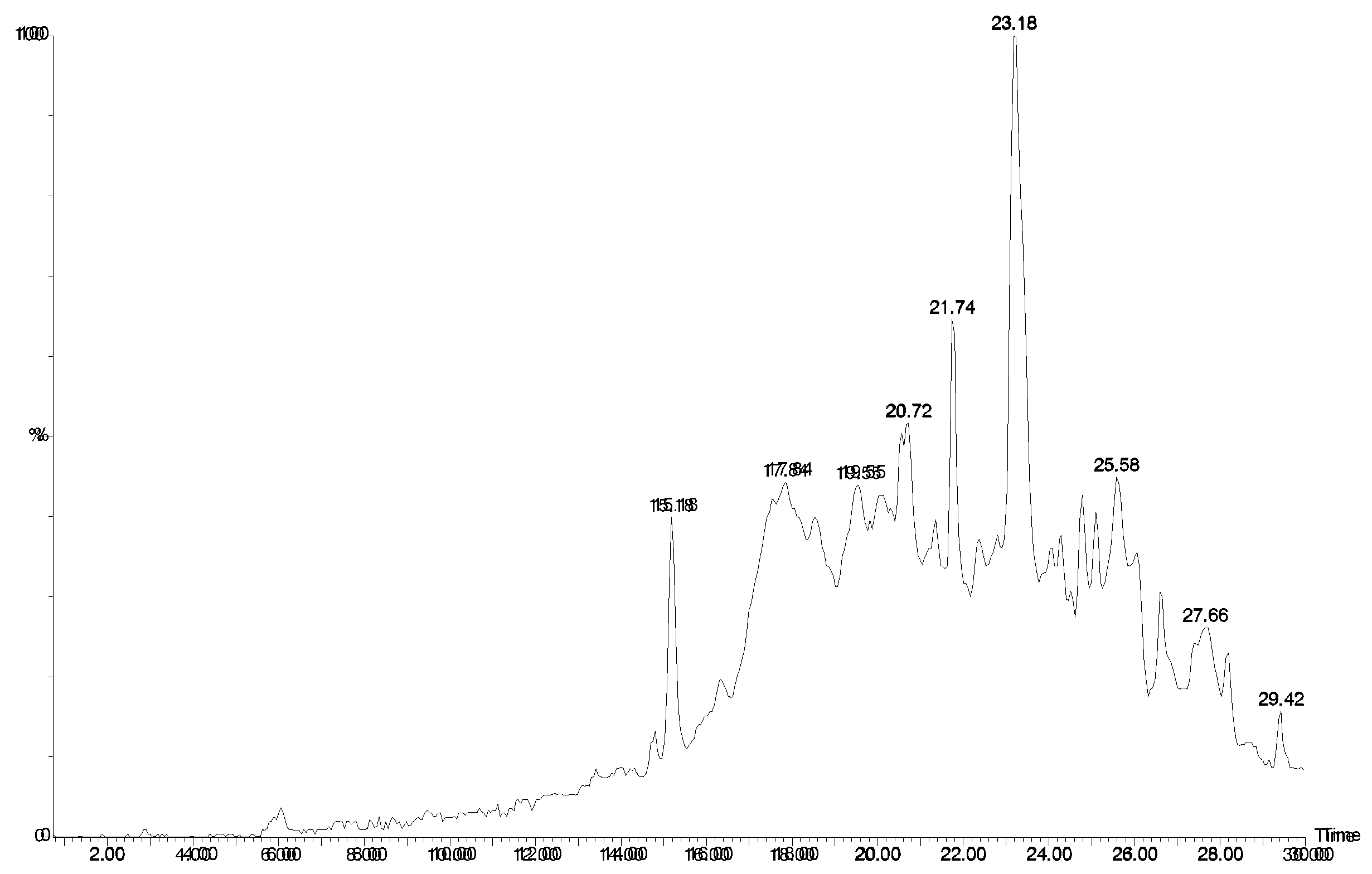
Figure 3.
HPLC chromatogram (UV 280 nm) of
Agelas sp. 1B. LCMS indicated essentially the same compounds were present as in
Agelas sp. 1A (
Table 1).
Figure 3.
HPLC chromatogram (UV 280 nm) of
Agelas sp. 1B. LCMS indicated essentially the same compounds were present as in
Agelas sp. 1A (
Table 1).
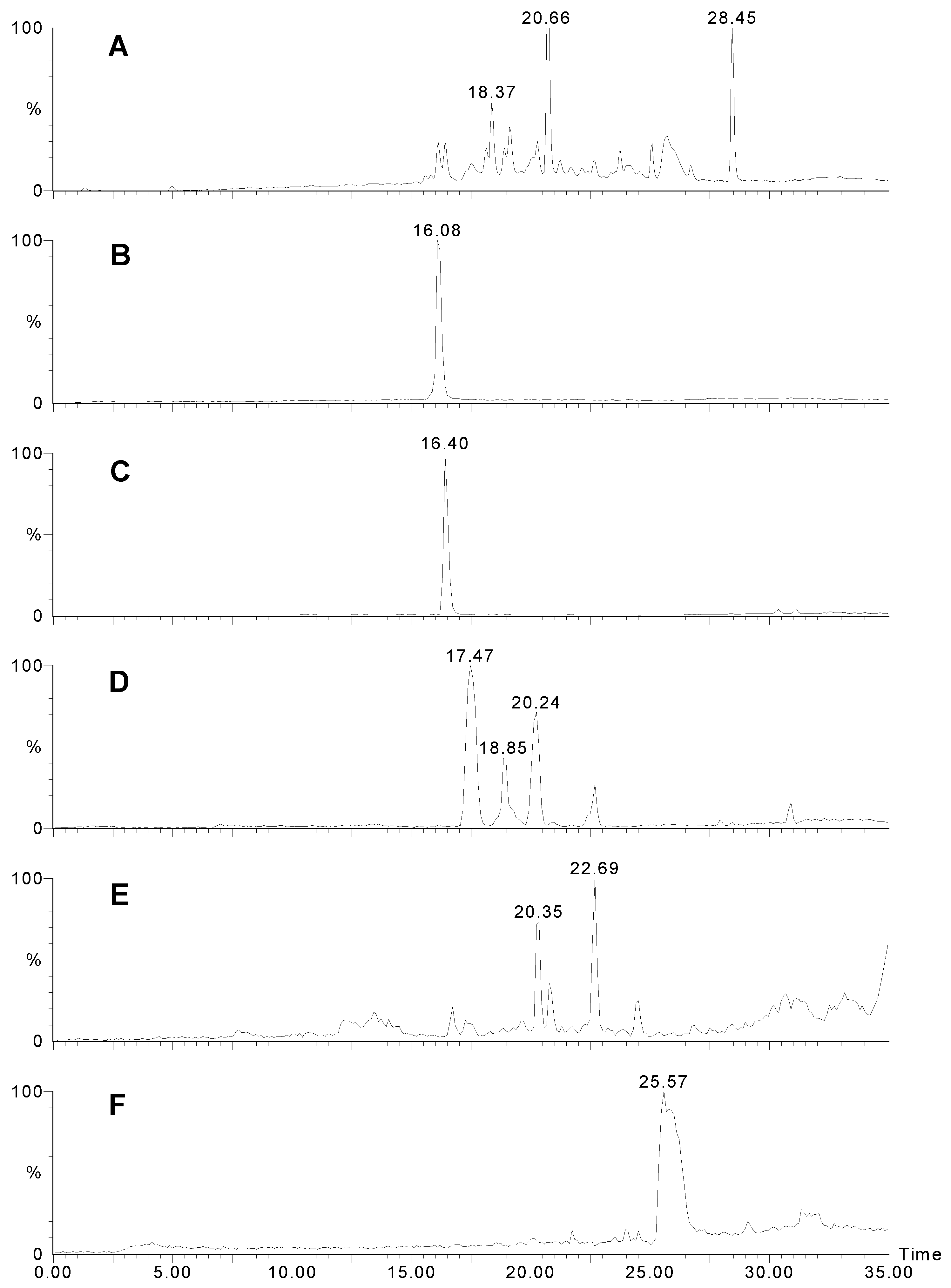
Figure 4.
LCMS traces for Agelas sp. 2. A) UV trace at 280 nm. B) Positive ion mass chromatogram of m/z 358. Manzacidine B elutes as a sharp peak. C) Positive ion mass chromatogram of m/z 344. D) Positive ion mass chromatogram of m/z 406. Note that there are several isomers with this weight. E) Positive ion mass chromatogram of m/z = 450. Unidentified compounds with two bromines. F) Positive ion mass chromatogram of m/z = 353 - possibly longamide B.
Figure 4.
LCMS traces for Agelas sp. 2. A) UV trace at 280 nm. B) Positive ion mass chromatogram of m/z 358. Manzacidine B elutes as a sharp peak. C) Positive ion mass chromatogram of m/z 344. D) Positive ion mass chromatogram of m/z 406. Note that there are several isomers with this weight. E) Positive ion mass chromatogram of m/z = 450. Unidentified compounds with two bromines. F) Positive ion mass chromatogram of m/z = 353 - possibly longamide B.
The five peaks eluting between oxysceptrin and nakamuric acid did not correspond to any known metabolite though each contained two bromine atoms and the peak at 16.6 min (m/z 679/681/683) is consistent with a monoacetate of sceptrin.
The same compounds were observed in
Agelas sp. 1B (
Fig 3). There were several other compounds present that contained two bromines and their molecular ions did not correspond to any compounds in our database of oroidin-like (or
Agelas spp) compounds.
Agelas sp. 2 contained completely different compounds (
Fig 4). The peaks eluting at 16.1 (
Fig. 4B) and 16.4 min. (
Fig. 4C) corresponded to compounds
5 of the manzacidine family [
12] where the R was a hydrogen (
m/z 344/346) or keto (
m/z 358/360 - a new compound). Structurally similar compounds; longamide A (
6) and B (
7) [
13] were also detected (18.4 and 25.6 min.;
Fig. 4F).
The other major compound (m/z 404/406/408; 17.5 min.;
Fig. 4D) contained two bromine atoms and its mass spectrum corresponds to dihydrospongiacidine (
8) or an isomer of which there are several known [
14]. Minor components included at least two isomers of 3-bromohymenialdosine (
8) (18.9 and 20.2 min.;
Fig. 4D; m/z 402/404/406) which could be E/Z isomers about the exocyclic double bond. Such compounds have been previously isolated from
Stylissa carteri [
15]. Several other unidentified compounds of this type were also observed and are likely to be new (eg MW 447;
Fig. 4E).
Finally,
Agelas cf
mauritiana, contained many compounds with bromine (
Fig. 5). Evidence for bromohymenialdisine and a hydrogenated forms (
8), eluting at 19.0 (
Fig. 5C) and 19.9/21.5 min. (
m/z 402/404/406 and 404/406/408;
Fig. 5B). The remaining compounds were so similar to
Agelas sp. 2 that we were led to conclude that they must be identical sponges.
Table 2.
Major peaks from LC-MS from Agelas sp. 2 and Agelas cf mauritiana.
Table 2.
Major peaks from LC-MS from Agelas sp. 2 and Agelas cf mauritiana.
| | Agelas sp. 2 | | |
| RT | Molecular ion | MW | Tentative structure |
| 16.1 | 358/360 | 357 | manzacidine B (5) |
| 16.4 | 346/348 | 345 | manzacidine A (5) |
| 17.5 | 404/406/408 | 403 | dihydrospongiacidine (8) |
| 18.4 | 309/311/313 | 308 | longamide A (6) |
| 18.9 | 402/404/406 | 401 | bromohymenialdisine (8) |
| 19.1 | 420/421/423 | 419 | |
| 20.2 | 402/404/406 | 401 | bromohymenialdisine isomer (8) |
| 20.3 | 448/450/452 | 447 | |
| 20.7 | 267/269/271 | 266 | 4,5-dibromopyrrole carboxamide |
| 22.7 | 448/450/452 | 447 | |
| 23.8 | 323/325/327* | 324 | |
| 25.4 | 379/381/383 | 378 | |
| 25.6 | 351/353/355 | 350 | longamide B (7) |
| 26.2 | 266/268/270* | 267 | 4,5-dibromopyrrole carboxylic acid |
| 28.5 | 294/296/298* | 295 | |
| | Agelas cf mauritiana | | |
| 17.5 | 346/348 | 345 | manzacidine A (5) |
| 19.0 | 404/406/408 | 403 | dihydrospongiacidine (8) |
| 19.4 | 309/311/313 | 308 | longamide A (6) |
| 19.9 | 402/404/406 | 401 | bromohymenialdisine (8) |
| 20.5 | 420/422/424 | 419 | |
| 21.5 | 402/404/406 | 401 | bromohymenialdisine isomer (8) |
| 21.6 | 448/450/452 | 447 | |
| 23.1 | 267/269/271 | 266 | 4,5-dibromopyrrole carboxamide |
| 23.8 | 446/448/450 | 267 | |
| 24.6 | 323/325/327* | 324 | |
| 25.9 | 365/367/369* | 366 | |
| 26.6 | 266/268/270* | 267 | 4,5-dibromopyrrole carboxylic acid |
| 29.2 | 294/296/298* | 295 | |
Figure 5.
LCMS analysis of Agelas cf mauritiana. A) HPLC UV trace (280 nm). B) mass selective HPLC trace at m/z 404 and C) m/z 406.
Figure 5.
LCMS analysis of Agelas cf mauritiana. A) HPLC UV trace (280 nm). B) mass selective HPLC trace at m/z 404 and C) m/z 406.
Experimental
Collection
Agelas sp. 1A (WAM Z1194), Agelas sp. 1B (WAM Z1193), and Agelas cf mauritiana (WAM Z1200) were all collected at Rat Island, Houtman Abrolhos Islands at 3-13m depth on 5 December 1996. All sponges were bright orange alive, thickly encrusting to mound shapes, and all occurred in semi-shade. WAM Z1194 was reproductive containing eggs WAM Z1193 was not reproductive, it was a slightly darker orange and slightly more contractile texture than Z1194. It also had more spicules in its primary fibres than Z1194, but this was not assessed quantitatively. Agelas sp. 2 (WAM Z17) was collected from Beacon Island, Houtman Abrolhos Islands. It was collected at ~20 m depth on 20 March 1997. It was not reproductive, was also an orange mound sponge, but had morphological, skeletal and spicule differences from the other material.
Analysis of morphology, colour and spiculation led to the conclusion that Z1194 and Z1193 were the same species but in one was a reproductively active female and the other specimen was not. The other two species were clearly different even though all were thickly encrusting orange sponges.
Mass spectrometry
Liquid chromatography-mass spectrometry was performed on a Quattro II, triple quadrupole mass spectrometer (Micromass, UK) fitted with an electrospray source. The RP-HPLC was performed on a HP 1090 LC using a Waters Nova-pak 4μ C18 (2 mm × 150 mm) column. UV detection was provided by a ABI Analytical Spectroflow 757 variable wavelength detector. The UV output was monitored at 280 nm and the UV trace was acquired by the Masslynx data system (Micromass) along with the MS data. A split placed after the UV detector directed approximately 20% of the flow to the mass spectrometer. Solvent A was MilliQ water, solvent B was UV grade methanol. A flow rate of 0.2 mL/min. was used. Two alternating mass spectrum scans were employed. In the positive ion mode the instrument was scanned from m/z 100 to m/z 1000 in 3 seconds at a cone voltage of 35 V. In the negative ion mode the instrument was scanned from m/z 120 to m/z 1000 in 3 seconds at a cone voltage of 30 V. The high voltage applied to the ESI capillary was 3.5 kV and 3.0 kV for the positive and negative ion modes respectively.