Synthesis of the New Type of Water-Soluble Ligand N,N-Bis-(diphenylphosphinomethyl)-2-aminoethylphosphonic Acid (Ph2PCH2)2NCH2CH2PO3H2 and Its Sodium Salt
Abstract
:Introduction
Results and Discussion.

| Reaction conditions | a | b | c | d | e |
|---|---|---|---|---|---|
| Yields (%) | 62.0 | 85.6 | 100 | 96.8 | 78.2 |
- (a)
- N(Et)3 and Na2CO3 absent, pH 4-5;
- (b)
- only N(Et)3 present, pH 7-8;
- (c)
- N(Et)3 and Na2CO3 present, pH 8.5-9.5;
- (d)
- N(Et)3 and Na2CO3 present, aqueous ethanol as solvent, pH 8.5-9.5;
- (e)
- NaOH present, pH=10.
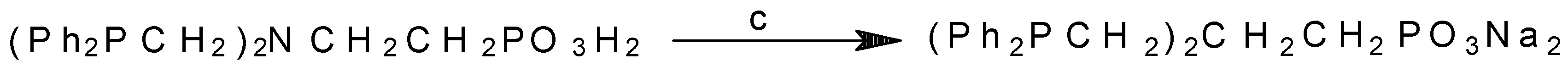
| Conditions | a | b | c | d |
| 31P NMR (≡P=O) | 37.71 | 37.70 | / | / |
| yields(%) | 75. 8 | 92.4 | 91.2 | 76.8 |
Experimental
General
Acknowledgements
References
- Papadogianakis, G.; Maat, L.; Sheldon, R.A. Catalytic Conversions in Water: a Novel Carbonylation Reaction Catalysed by Palladium Triphenylphosphine Complexes. J. Chem. Soc. Chem. Commun. 1994, 2659. [Google Scholar] [CrossRef]
- Avey, A.; Schut, D.M.; Weakley, T.J.R. A New Water-soluble Phosphine for Use in Aqueous Organometallic System: Products for the Reactions of 2,3-Bis(diphenylphosphino)maleic Anhydride with Water and Oxygen. Inorg. Chem. 1993, 32, 233. [Google Scholar] [CrossRef]
- Cornils, B.; Herrmann, W.A. ”Aqueous-phase organometallic catalysis”. Wiley-VCH: Weinhein, 1998. [Google Scholar]
- Ravindar, V.; Hemling, H.; Schumann, H.; Blum, J. A New Synthesis of Hydrophilic Carboxylated Arylphosphines. Synth. Commun. 1992, 22, 841. [Google Scholar] [CrossRef]
- Ellis, J.W.; Harrison, K.N.; Hoye, P.A.T. Water-soluble Tri(hydromethyl)phosphine Complexes. Inorg. Chem. 1992, 31, 3026. [Google Scholar] [CrossRef]
- Mitchell, T. N.; Heeshe-Wagner, K. Approach to New Water-Soluble Phosphines. J. Organomet. Chem. 1992, 436, 43. [Google Scholar] [CrossRef]
- Renaud, E.; Russell, R.B.; Fortier, S.; Brown, S.J.; Baird, M.C. Synthesis of A New Family of Water-soluble Tertiary Phosphine Ligands in Aqueous and Biphasic Media. J. Organomet. Chem. 1991, 419, 403. [Google Scholar] [CrossRef]
- Smith, R.T.; Ungar, R.K.; Sanderson, L.J. Rhodium Complexes of the Water-Soluble Phosphine Ph2PCH2CH2NMe3+. Organometallics 1983, 2, 1138. [Google Scholar] [CrossRef]
- Schull, T.L.; Fettinger, J. C.; Knight, D. A. Synthesis and Characterization of Palladium(I) and Platinum(II) complexes Containing Water-Soluble Hybrid Phosphine-Phosphonate Ligands. Inorg. Chem. 1996, 35, 6717. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Moriyama, Y.; Konishi, H. “Counter” Phase Transfer Catalysis by Water-soluble Phosphine Complexes. Chem. Lett. 1986, 1463. [Google Scholar] [CrossRef]
- Fu, X.K.; Gong, C. B.; Ma, X.B. A New Simple Route to N-substituted 2-Aminoethylphosphonic acids. Synth. Commun. 1998, 28, 2659. [Google Scholar] [CrossRef]
- Digiacomo, P.M.; Dines, M. B.; Parziale, V.E. Layered or Amorphous Organometallic Inorganic Polymers and Their Use. Eur. Pat. Appl. 1980, 10 857, CA: 1981, 94, 103554d. [Google Scholar]
- Fawcett, J.; Hoye, P. A. T.; Kemmitt, R.D.W. Synthesis of Bis(phosphinomethyl)amines Via Bis- (hydroxymethyl)phosphonium Salts. J. Chem. Soc. Dalton Trans. 1993, 2563. [Google Scholar] [CrossRef]
- Kiss, T.; Balla, J.; Nagy, G. Complexes of Aminophosphonates. Inorg. Chimica Acta 1987, 138, 25. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Ma, X.; Fu, X.; Li, L.; Chen, J. Synthesis of the New Type of Water-Soluble Ligand N,N-Bis-(diphenylphosphinomethyl)-2-aminoethylphosphonic Acid (Ph2PCH2)2NCH2CH2PO3H2 and Its Sodium Salt. Molecules 2001, 6, 390-395. https://doi.org/10.3390/60400390
Ma X, Fu X, Li L, Chen J. Synthesis of the New Type of Water-Soluble Ligand N,N-Bis-(diphenylphosphinomethyl)-2-aminoethylphosphonic Acid (Ph2PCH2)2NCH2CH2PO3H2 and Its Sodium Salt. Molecules. 2001; 6(4):390-395. https://doi.org/10.3390/60400390
Chicago/Turabian StyleMa, Xuebing, Xiangkai Fu, Longqin Li, and Jingrong Chen. 2001. "Synthesis of the New Type of Water-Soluble Ligand N,N-Bis-(diphenylphosphinomethyl)-2-aminoethylphosphonic Acid (Ph2PCH2)2NCH2CH2PO3H2 and Its Sodium Salt" Molecules 6, no. 4: 390-395. https://doi.org/10.3390/60400390




