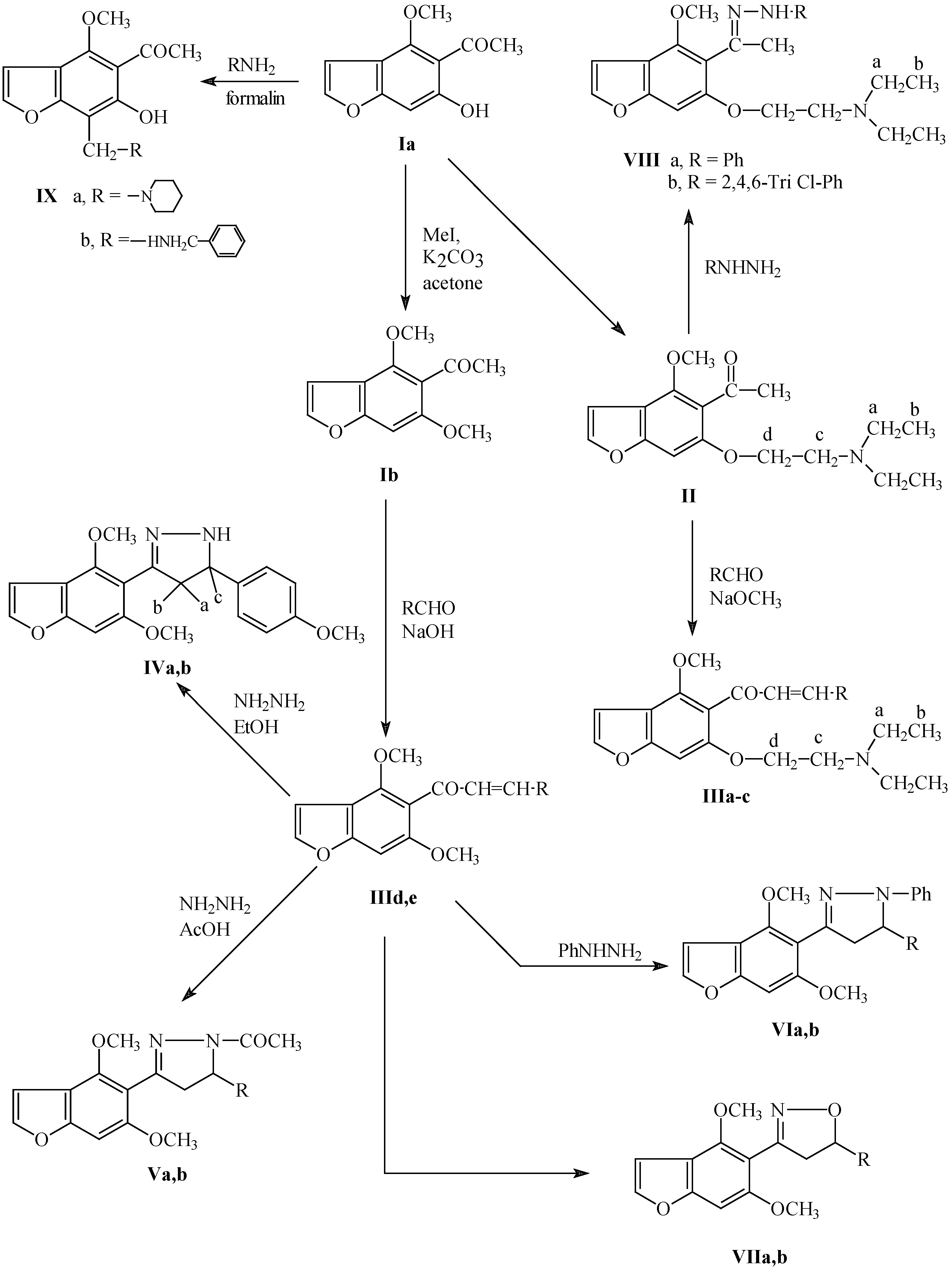
Experimental
General
Melting points are uncorrected.
1H-NMR spectra were run using TMS as internal reference on a Jeol EX-270 NMR spectrometer. IR spectra were recorded on a FT/IR Jasco 300 E instrument. The prepared compounds were analyzed for C, H and N and the microanalytical data is in full agreement with the suggested structures (
Table 1). Compound
Ia was prepared according to Musante [
13].
Compound
Ib was prepared according to Schonberg [
14] and compound
IXa was prepared according to Ragab [
7]. The biological activity of the prepared compounds is under investigation and will be published separately in near future.
Scheme 1.
III-VII, a,d, R = Ph; b,e, R = C6H4-OCH3 (p); c, R = C6H4-Cl (p)
Preparation of 5-acetyl-6-diethylaminoethoxy-4-methoxybenzo[b]furan (II).
A mixture of visnaginone (0.2 mole), potassium carbonate (5.4g) and diethylaminoethyl chloride (0.47 mole) in acetone (340 mL) was refluxed with stirring for 10 hrs. The acetone mixture was filtered off and filtrate was evaporated under reduced pressure, and extracted with chloroform, the chloroform washed with sodium hydroxide solution (5%), then with water, the chloroform extract was dried over anhydrous sodium sulphate and evaporated under reduced pressure to give 5-acetyl-6-diethylamino-ethoxy benzofuran (II) as yellowish white oil. IR (KBr) cm-1 2950 (CH-alkyl), 1750 (C=O), 1225 (C-N) and 1150 (C-O-C); 1H-NMR (CDCl3) ppm: δ 1.05 (6H, t, CH3-b), 2.45 (3H, s, COCH3), 2.55 (4H, q, 2CH2-a), 2.8 (2H, t, CH2-c), 2.95 (2H, t, CH2-d), 4.00 (3H, s, OCH3), 6.75 (1H, s, C-7 aromatic), 6.9 (1H, d, H3-furan) and 7.45 (1H, d, H2-furan).
General procedure for the preparation of 5-substituted cinnamoyl-6-(2-diethylaminoethoxy)-4-methoxy benzo[b]furans (IIIa-c).
Compound II (0.3 mole) was reacted with a mixture of the appropriate aromatic aldehyde (0.3 mole), dry methanol (30 mL) and sodium methoxide (from 3g Na and 30 mL methanol) with stirring at room temperature for 4 hrs., then water (750 mL) was added and the mixture extracted with ethyl acetate. The organic layer washed with water, dried over anhydrous sodium sulphate, concentrated and crystallized from methanol.
5-cinnamoyl-6-(diethylaminoethoxy)-4-methoxybenzo[b]furan (IIIa): Yield 95%, m.p. 110°C. IR: (KBr) cm-1, 3000 (C-H), 1730 (C=O), 1615 (cinnamoyl C=C) and 1135 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ at 1.0 (6H, t, CH3-a), 2.45 (4H, q, CH2-b), 2.7 (2H, t, CH2-c), 3.57 (2H, t, CH2-d), 4.0 (3H, s, OCH3 aromatic), 4.20 (3H, s, OCH3-C4), 6.9-7.4 (6H, m, benzene ring), 7.55 (1H, d, H3 furan) and 7.7 (1H, d, H2-furan).
5-(4-Methoxycinnamoyl-6-(diethylaminoethoxy)-4-methoxybenzo[b]furan (IIIb): Yield 90%, m.p. 116°C. IR: (KBr) cm-1, 3110 (C-H), 1620 (cinnamoyl C=C), 1635 (C=O) and 1130 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ 0.9 (6H, t, CH3-a), 2.4 (4H, q, CH2-b), 2.6 (2H, t, CH2-c), 3.5 (2H, t, CH2-d), 4.00 (3H, s, OCH3-aromatic), 4.10 (3H, s, OCH3-C4), 7.2, 7.9 (1H, dd, 2H-cinnamoyl), 6.95, 7.40 (4H, dd, aromatic protons), 7.45 (1H, s, C-7 aromatic), 7.5 (1H, d, H3-furan), and 7.7 (1H, d, H2-furan).
5-(4-Chlorocinnamoyl-6-(diethylaminoethoxy)-4-methoxybenzo[b]furan (IIIc): Yield 85%, m.p. 209°C. IR: (KBr) cm-1, 3050 (C-H), 1600 (cinnamoyl C=C), 1700 (C=O), 1120 (C-O-C), 1220 (C-N) and 730 (C-Cl); 1H-NMR (DMSO-d6) ppm: δ 0.95 (6H, t, CH2-a), 2.4 (4H, q, CH2-b), 2.75 (2H, t, CH2-c), 3.3 (2H, t, CH2-d), 4.00 (3H, s, OCH3), 6.8-7.3 (6H, m, aromatic protons + aliphatic system), 7.5 (1H, s, H-7), 7.8 (1H, d, H-3, furan) and at 7.9 (1H, d, H-2 furan).
General procedure for the preparation of 5-substituted cinnamoyl-4,6-dimethoxybenzo[b]furans (IIId,e).
6-Methoxy visnaginone Ib (0.1 mole) was dissolved in ethyl alcohol (15 mL). The appropriate aromatic aldehyde (0.1 mole) was added, followed by the addition of a solution of sodium hydroxide (30%, 12 mL). The mixture was stirred and allowed to stand at room temperature for 24 hrs., then diluted with water (270 mL) and acidified with dilute solution HCl. The precipitate formed was filtered off and crystallized from ethyl alcohol.
5-Cinnamoyl-4,6-dimethoxybenzo[b]furan (IIId): Yield 97%, m.p. 106°C. IR: (KBr) cm-1, 3000 (C-H), 1680 (C=O), 1620 (C=C) and 1165 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ 3.8 (2H, s, OCH3-C4), 3.95 (3H, s, OCH3-C4), 6.8 (1H, d, H3 furan), 6.9, 7.6 (2H, dd, 2H cinnamoyl), 7.1-7.5 (5H, m, aromatic), 7.8 (1H, d, H-3, furan) and at 7.9 (1H, d, H-2 furan).
5-(4-Methoxycinnamoyl-6-(diethylaminoethoxy)-4-methoxybenzo[b]furan (IIIe): Yield 90%, m.p. 108°C. IR: (KBr) cm-1, 3040 (C-H), 1700 (C=O), 1625 (C=C) and 1150 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ at 0.90 (6H, t, CH3-a), 2.4 (4H, q, CH2-b), 2.60 (2H, t, CH2-c), 3.5 (2H, t, CH2-d), 4.00 (3H, s, OCH3 aromatic), 4.10 (3H, s, OCH3-C4), 7.2, 7.7 (1H, dd, 2H cinnamoyl), 6.95-7.4 (5H, m, aromatic protons), 7.45 (1H, s, H-7, aromatic), 7.5 (1H, d, H-3, furan) and at 7.9 (1H, d, H-2 furan).
General procedure for the preparation of 4,6-dimethoxy-5-(aryl-2-pyrazolin-3-yl)benzo[b]furans (IVa,b).
Chalcones IIId,e (0.01 mole) were dissolved in ethyl alcohol (10 mL) and refluxed with hydrazine hydrate (0.5 mL) for 10 hrs. The reaction mixture was diluted with water (50 mL) and the solid formed was filtered off and crystallized from ethyl alcohol.
4,6-Dimethoxy-5-(phenyl-3-pyrazolin-3-yl)benzo[b]furan (Iva): Yield 55%; m.p. 131°C. IR: (KBr) cm-1, 3230 (NH), 1620 (C=C), 1590 (C=N) and 1150 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ 2.8 (1H, dd, Ha, Jvic = 8 Hz, Jgem=15 Hz), 3.6 (1H, dd, He, Jvic = 8 Hz, Jgem = 10 Hz), 3.9 (3H, s, OCH3, C-6), 4.00 (3H, s, OCH3-C-4), 5.4 (1H, dd, Hc pyrazolinyl, Jvic = 10 Hz, Jgem = 15 Hz), 6.6 (1H, d, H3-furan), 6.95-7.40 (4H, m, aromatic), 7.5 (1H, s, C-7), 7.9 (1H, d, H2-furan), and 9.6 (1H, s, NH, exchangeable with D2O).
4,6-Dimethoxy-5-(4-methoxyphenyl-2-pyrazolin-3-yl)benzo[b]furan (IVb): Yield 60; m.p. 64°C. IR: (KBr) cm-1, 3210 (NH), 1700 (C=O), 1625 (C=C), 1590 (C=N) and 1155 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ 2.9 (1H, dd, Ha), 3.6 (1H, dd, He), 3.8 (3H, s, OCH3, aromatic), 3.9 (3H, s, OCH3-C-6), 4.0 (3H, s, OCH3, C-4), 5.4 (1H, dd, He pyrazolinyl), 6.65 (1H, d, H3-furan), 6.95, 7.65 (4H, dd, aromatic), 7.5 (1H, s, C-7), 7.9 (1H, d, H2-furan), and 9.6 (1H, s, NH, exchangeable with D2O).
General procedure for the preparation of 4,6-dimethoxy-5-(1-acetyl-5-aryl-2-pyrazolin-3-yl)benzo[b]furans (Va,b).
Chalcones IIId,e (0.01 mole) were dissolved in acetic acid (10 mL) and refluxed with hydrazine hydrate (0.5 mL) for 12 hrs. The reaction mixture was poured onto water. The solid formed was filtered off and crystallized from ethanol.
4,6-Dimethoxy-5-(1-acetylphenyl-2-pyrazolin-3-yl)benzo[b]furan (Va): Yield 90%; m.p. 170°C. IR: (KBr) cm-1, 1670 (keto amide), 1635 (C=N) and observed disappearance of NH band; 1H-NMR (DMSO-d6) ppm: δ 2.2 (3H, s, COCH3), 2.7 (1H, dd, Ha, Jvic = 6 Hz, Jgem= 18 Hz), 3.5 (1H, dd, He, Jvic = 6 Hz, Jgem = 12 Hz), 3.9, 4.00 (3H, s, OCH3, C-4,6), 6.0 (1H, dd, Hc pyrazolinyl, Jvic = 12 Hz, Jgem = 18 Hz), 6.9 (1H, d, H3-furan), 7.1-7.3 (5H, m, aromatic), 7.6 (1H, s, C-7) and 7.95 (1H, d, H2-furan).
4,6-Dimethoxyacetyl-5-(4-methoxyphenyl-2-pyrazolin-3-yl)benzo[b]furan (Vb): Yield 85%; m.p. 170°C. IR: (KBr) cm-1, 1660 (keto amide), 1635 (C=N); 1H-NMR (DMSO-d6) ppm: δ 2.1 (3H, s, N-COCH3), 2.8 (1H, dd, Ha), 3.6 (1H, dd, Hc), 3.75 (3H, s, OCH3-aromatic), 3.9, 4.00 (3H, s, OCH3, C-4,6), 5.9 (1H, dd, He), 6.9 (1H, d, H3-furan), 7.1-7.3 (4H, m, aromatic), 7.4 (1H, s, C-7), and 7.95 (1H, d, H2-furan).
General procedure for the preparation of 4,6-dimethoxy-5-(5-aryl-1-phenyl-2-pyrazolin-3-yl)benzo[b]furans (VIa,b).
Chalcones IIId,e, (0.01 mole) were dissolved in glacial acetic acid (10 mL) and refluxed with phenyl hydrazine (1.1 mL) for 8 hrs. The reaction mixture was cooled and poured onto water. The precipitate thus formed was filtered off and crystallized from ethyl alcohol.
4,6-Dimethoxy-5-(5-diphenyl-2-pyrazolin-benzo[b]furan (VIa): Yield 75%; m.p. 120°C; 1H-NMR (DMSO-d6) ppm: δ 2.8 (1H, dd, Ha, Jvic = 8 Hz, Jgem= 18 Hz), 3.6 (1H, dd, He, Jvic = 12 Hz, Jgem = 18 Hz), 3.8 (3H, s, OCH3, C-6), 400 (3H, s, OCH3, C-4), 5.85 (1H, dd, Hc, Jvic = 8 Hz, Jgem = 18 Hz), 6.95 (1H, d, H3-furan), 7.2-7.5 (10H, m, aromatic), 7.6 (1H, s, C-7) and 7.9 (1H, d, H2-furan).
4,6-Dimethoxy-1-phenyl-5-(4-methoxyphenyl)pyrazolin-3-yl)-benzo[b]furan (VIb): Yield 75% (ethanol), m.p. 120°C. 1H-NMR (DMSO-d6) ppm: δ 2.9 (1H, dd, Ha), 3.7 (1H, dd, He), 3.75 (3H, s, OCH3-aromatic), 3.8 (3H, s, OCH3-C-6), 4.00 (3H, s, OCH3-C-4), 5.85 (1H, dd, Hc), 6.9 (1H, d, H3-furan), 7.1-7.4 (9H, m, aromatic), 7.45 (1H, s, C-7), 7.9 (1H, d, H2-furan).
General procedure for the preparation of 4,6-dimethoxy-5-(5-aryl-2-isoxazolin-3-yl)benzo[b]furans (VIIa,b).
Chalcones IIId,e (0.01 mole) were dissolved in ethanol (10 mL) and a mixture of hydroxylamine hydrochloride (0.8g) in ethanol (8 mL) and water (2 mL) was added, followed by few drops of potassium hydroxide (50%). The reaction mixture was refluxed for 9 hrs. The solid formed was filtered off and crystallized from ethyl alcohol.
4,6-Dimethoxy-5-(phenyl-2-isoxazolin-3-yl)benzo[b]furan (VIIa): Yield 80%; m.p. 138°C; IR: (KBr) cm-1, 1635 (C=N), 1600 (C=C), and 1030 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ at 2.45 (1H, dd, Ha, Jvic = 6 Hz, Jgem= 18 Hz), 3.8, 3.9 (3H, s, OCH3, C-4,6), 4.1 (1H, dd, He, Jvic = 6 Hz, Jgem = 12 Hz), 5.5 (1H, dd, Hc, Jvic = 12 Hz, Jgem = 18 Hz), 6.9 (1H, d, H3-furan), 7.2-7.4 (5H, m, aromatic), 7.5 (1H, s, C-7) and 7.9 (1H, d, H2-furan).
4,6-Dimethoxy-5-(4-methoxyphenyl)-2-isoxazolin-3-yl)benzo[b]-furan (VIb): Yield 70%; m.p. 70°C. IR: (KBr) cm-1, 1635 (C=N), 1620 (C=C), and 1030 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ 2.4 (1H, dd, Ha), 3.8 (3H, s, OCH3, aromatic), 3.9, 4.00 (3H, s, OCH3-C-4,6), 4.3 (1H, dd, He), 5.5 (1H, dd, Hc), 6.8 (1H, d, H3-furan), 7.1-7.4 (4H, dd, aromatic, J = 8 Hz), 7.45 (1H, s, C-7), 7.9 (1H, d, H2-furan).
General procedure for the preparation of 5-(1-arylhydrazono-ethyl)-6-(2-diethylaminoethoxy)-4-methyl benzo[b]furans (VIIIa,b).
Compound II (0.01 mole) was dissolved in ethyl alcohol (10 mL), 0.1 mole of a hydrazine was added (phenyl hydrazine in case of VIIIa and 2,4,6-trichlorophenyl hydrazine in case of VIIIb), followed by the addition of few drops of acetic acid and the reaction mixture was refluxed for 5 hrs. and then cooled. The solid material was filtered off and crystallized from ethyl alcohol.
5-(2-Phenyhydrazonethyl)-6-diethylaminoethyl)-4-methoxy-benzo[b]furan (VIIIa): Yield 70%; m.p. 125°C; IR: (KBr) cm-1, 3150 (NH), 1630 (C=C), 1597 (C=N), 1320 (C-N), and 1106 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ 0.9 (6H, t, 2CH3-b), 2.5 (4H, q, 2CH2-a), 3.3 (2H, t, 2CH2-d), 3.9 (3H, s, OCH3), 7.1 (1H, d, H3-furan), 7.4 (5H, m, aromatic), 7.6 (1H, s, C-7), 7.7 (1H, d, H2-furan), 10.0 (1H, s, NH exchangeable with D2O).
5-(2,4,6-Trichloro-1-phenyhydrazonoethyl)-6-(diethylamino-ethyl)-4-methoxybenzo[b]furan (VIIIb): Yield 75%; m.p. 130°C; IR: (KBr) cm-1, 3175 (NH), 1630 (C=C), 1597 (C=N), 1320 (C-N), and 1106 (C-O-C); 1H-NMR (DMSO-d6) ppm: δ 1.00 (6H, t, 2CH3-b), 2.6 (4H, q, 2CH2-a), 3.2 (2H, t, CH2-d), 3.9 (3H, s, OCH3), 7.2 (1H, d, H3-furan), 7.4 (2H, s, aromatic), 7.6 (1H, s, C-7), 7.7 (1H, d, H2-furan), 10.0 (1H, s, NH, exchangeable with D2O).
General procedure for the preparation of 5-acetyl-6-hydroxy-4-methoxy-7-substituted methyl benzo[b]furans (IXa,b).
Visnaginone (Ia, 0.01 mole) was dissolved in ethyl alcohol (20 mL). Formalin (0.5 mL) and the appropriate amine (0.01 mole) were added. The reaction mixture was refluxed for 3 hrs., and then cooled. The precipitate formed was filtered off and crystallized from ethyl alcohol.
5-Acetyl-6-hydroxy-4-methoxy-7-piperidinomethylbenzo[b]furan (IXa): Yield 80%; m.p. 105°C; IR: (KBr) cm-1, 3400 (broad OH), 3200 (NH), and 1320 (C-N); 1H-NMR (DMSO-d6) ppm: δ 2.3 (CH2-N), 3.9 (3H, s, COCH3), 4.0 (3H, s, OCH3), 4.8-5.2 (10H, m, piperidine), 6.9 (1H, d, H3-furan), 7.9 (1H, d, H2-furan), 12.1 (1H, bs, OH).
5-Acetyl-6-hydroxy-4-methoxy-7-benzylaminoethylbenzo[b]furan (IXb): Yield 90%; m.p. 120°C; IR: (KBr) cm-1, 3400 (broad OH), 3150 (NH), 1350 (C-N); 1H-NMR (DMSO-d6) ppm: δ at 2.4 (2H, s, CH2-N), 2.6 (2H, s, CH2-Ph), 3.9 (3H, s, COCH3), 4.00 (3H, s, OCH3), 6.9 (1H, d, H3-furan), 7.1-7.3 (5H, m, aromatic), 7.9 (1H, d, H2-furan), 12.1 (1H, br.s, OH).
Table 1.
Physical data for the prepared benzo[b]furan (IIIa-IXb)
Table 1.
Physical data for the prepared benzo[b]furan (IIIa-IXb)
| No. | X
Y
Z | M.P. °C (Yield,%) | Mol. Formula Mol. wt. | CHN Analysis % Calc.-Found |
|---|
| IIIa | Cinnamoyl
2-diethylaminoethyl
H | 110
95 | C24H27NO4
393 | 73.28
6.87
3.56 | 73.45
6.62
3.25 |
| IIIb | p-methoxycinnamoyl
2-diethylaminoethyl
H | 116
90 | C25H29NO5
423 | 70.92
6.86
3.31 | 70.73
6.91
3.16 |
| IIIc | 4-chlorocinnamoyl
2-diethylaminoethyl
H | 190
90 | C24H26NO4Cl
427.5 | 67.37
6.08
3.27 | 67.13
6.24
3.43 |
| IIId | cinnamoyl
methyl
H | 106
97 | C19H16O4
308 | 74.03
5.19 | 74.35
5.28 |
| IIIe | p-methoxycinnamoyl
methyl
H | 108
90 | C20H18O5
338 | 71.06
5.33 | 70.52
5.17 |
| IVa | 5-phenyl-2-pyrazolin-3-yl
methyl
H | 131
55 | C19H18N2O3
322 | 70.81
5.59
8.69 | 70.54
5.36
8.35 |
| IVb | 4-methoxyphenyl-2-pyrazolin-3-yl
methyl
H | 64
60 | C20H20N2O4
352 | 68.18
5.68
7.95 | 68.39
5.79
8.24 |
| Va | 1-acetylphenyl-2-pyrazolin-3-yl
methyl | 170
90 | C21H20N2O4
364 | 69.23
5.49
7.69 | 69.51
5.61
7.93 |
| Vb | 1-acetyl-5-(4-methoxy-phenyl)pyrazolin-3-yl
methyl | 173
85 | C22H22N2O5
394 | 67.00
5.58
7.10 | 67.26
5.36
7.38 |
| VIa | 1,5-diphenyl-2-pyrazolin-3-yl
methyl
H | 121
70 | C25H22N2O3
398 | 75.38
5.53
7.04 | 75.16
5.29
7.32 |
| VIb | 1-phenyl-5-(4-methoxy phenyl)-2-pyrazolin-3-yl
methyl
H | 120
75 | C26H24N2O4
428 | 72.89
5.61
6.54 | 72.53
5.89
6.98 |
| VIIa | 5-phenyl-2-isoxazoline-3-yl
methyl
H | 138
80 | C19H17NO4
323 | 70.59
5.26
4.33 | 70.28
4.83
4.11 |
| VIIb | 5-(4-methoxyphenyl)-2-isoxazoline-3-yl
methyl
H | 102
70 | C20H19NO5
353 | 67.99
5.38
3.97 | 67.68
5.21
3.71 |
| VIIIa | 2-phenylhydrazonoethyl
2-diethylaminoethyl
H | 125
70 | C23H29N3O3
395 | 69.87
7.34
10.63 | 69.71
7.26
10.41 |
| VIII b | 2,4,6-trichloro-1-phenyl-hydrazonoethyl
2-diethylaminoethyl
H | 130
75 | C23H26N3O3Cl3
498.5 | 55.37
5.22
8.42 | 55.51
5.35
8.66 |
| IXa | acetyl
H
piperidinomethyl | 105
80 | C17H21NO4
303 | 67.33
6.93
4.62 | 67.76
6.84
4.38 |
| IXb | acetyl
H
benzylaminoethyl | 120
90 | C19H19NO4
325 | 70.15
5.85
4.31 | 75.38
5.98
4.71 |