Synthesis and Characterization of Two New p-tert-Butylcalix[4]-arene Schiff Bases
Abstract
:Introduction
Results and Discussion
Synthesis of the Schiff Bases

| Compound | Formula Weight | Color | m.p, (oC) | Yield, % | Calcd. (Found) % | ||
| C | H | N | |||||
| H2L1.H2O (C56H73NO6) | 838.17 | Yellow | 192 | 86 | 80.25(79.42) | 8.54(8.69) | 1.67(1.44) |
| HL2 (C59H77NO5) | 880.25 | Yellow | 172 | 84 | 80.50(80.74) | 8.82(8.79) | 1.59(1.88) |
IR Spectra
| Compound | ν (H2O) | ν (O-H) | ν (C-H) | ν (C=N) |
| H2L1.H2O | 3420 mbr | 3547 | 2960, 2874 s | 1620 |
| HL2 | - | 3540 | 2960, 2875 s | 1620 |
Electronic Spectra
| Compound | ν ( cm-1) | Δν( cm-1) | λex( nm ) (excitation) | λem.( nm) (emission) | |
| CHCl3 | CH3CN | ||||
| H2L1 | 277 | 281 | 400 | 390 | 526 |
| HL2 | 287 | 290 | 300 | 390 | 522 |
1H-NMR Spectra
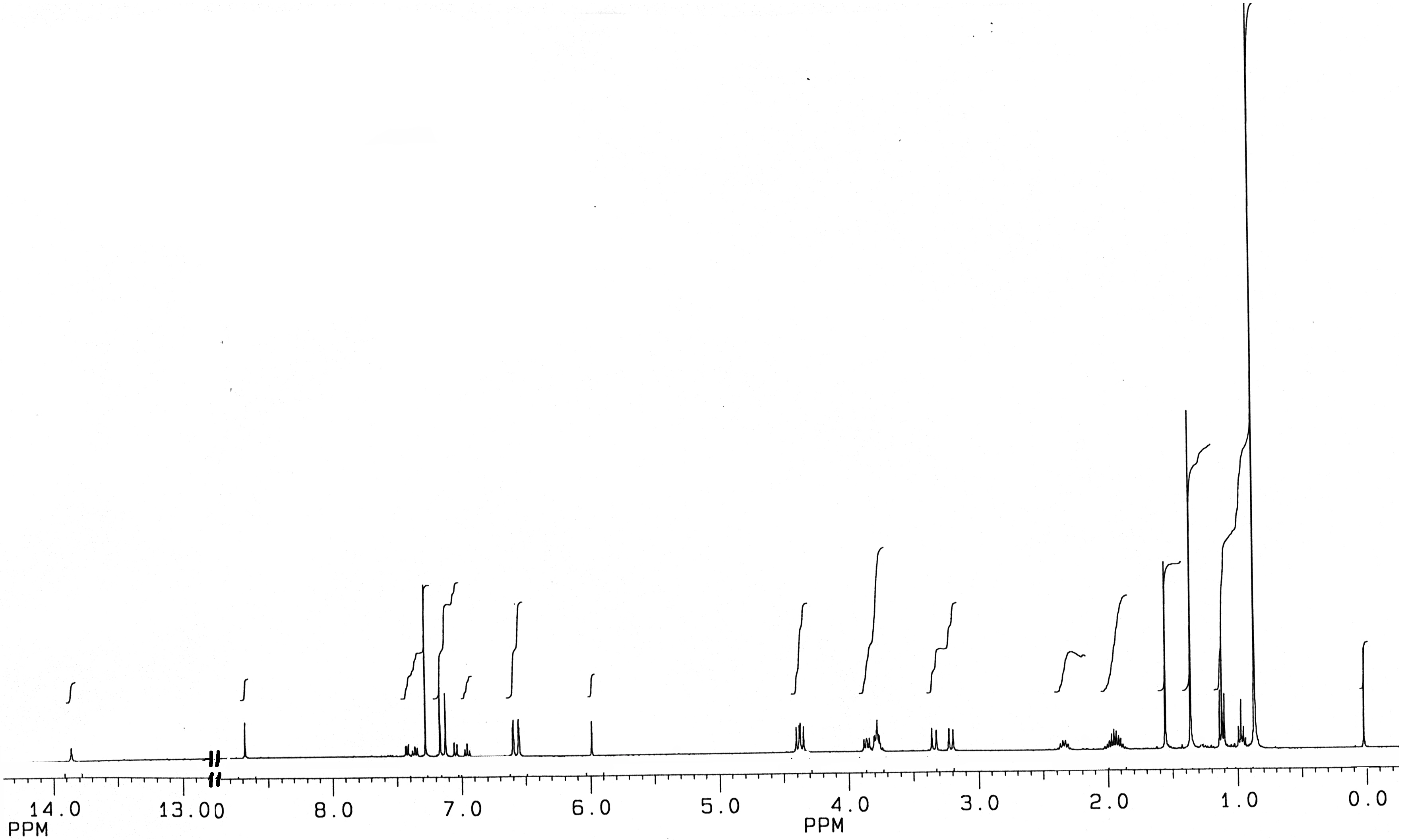
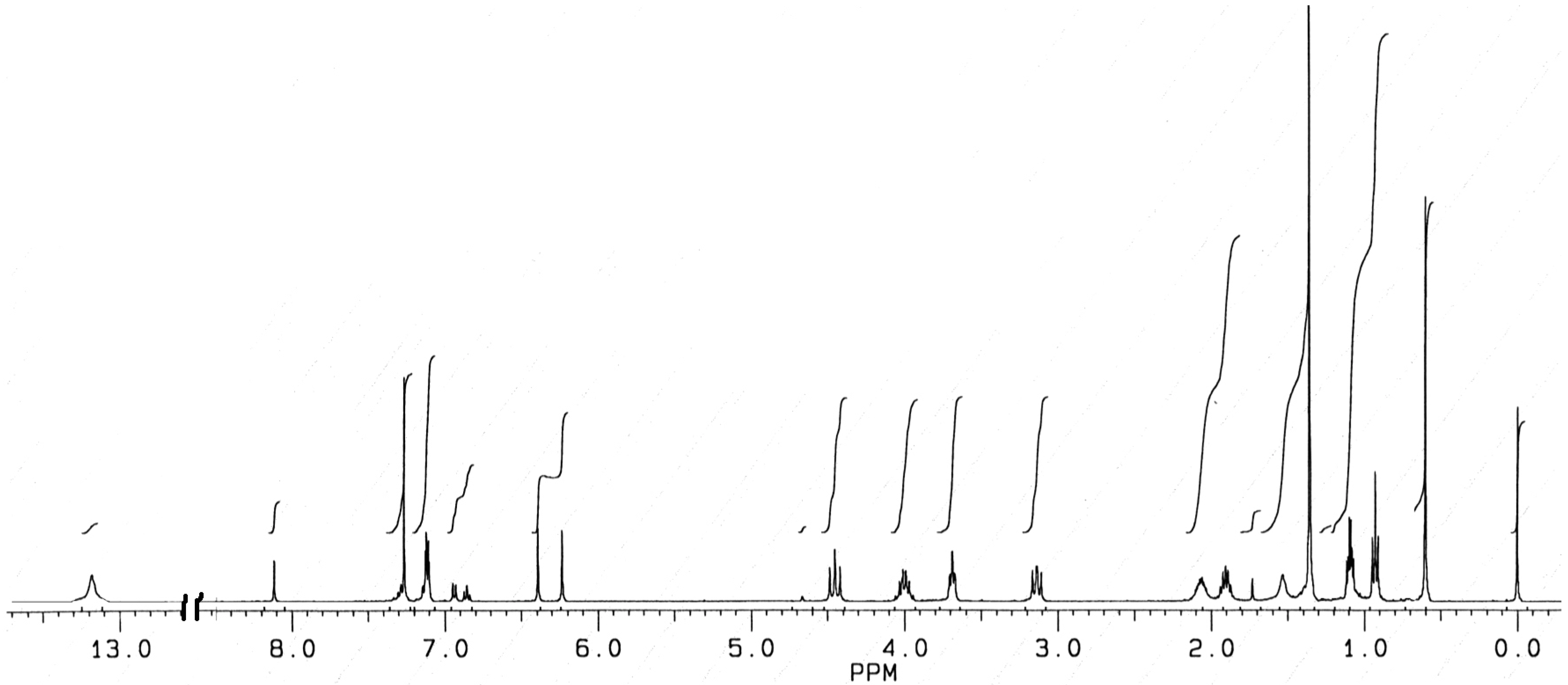
Conclusions
Experimental
General
Preparation of H2L1 and HL2
Acknowledgements
References
- Gutche, C. D. Calixarenes, Revisited; The Royal Society of Chemistry: Cambridge, 1998. [Google Scholar]
- Vicens, J.; Boehmer, V. Calixarenes: A Versatile Class of Macrocyclic Compounds; Kluver Academic: Boston, 1991. [Google Scholar]
- Van Loon, J. D.; Arduini, A.; Coppi, L.; Verboom, W.; Pochini, A.; Ungaro, R.; Harkema, S.; Reinhoudt, D. N. J. Org. Chem. 1990, 55, 5639. [CrossRef]
- Morzherin, Y.; Rudkevich, D. M.; Verboom, W.; Reinhoudt, D. N. J. Org. Chem. 1993, 58, 7602. [CrossRef]
- Casnati, A.; Pochini, A.; Ungaro, R.; Ugozzoli, F.; Arnaud, F.; Fanni, S.; Schwing, M. J.; Egberink, R. J. M.; Dejong, F.; Reinhoult, D. N. J. Am. Chem. Soc. 1995, 117, 2767. [CrossRef]
- Arnaud-Neu, F.; Fanni, S.; Guerra, L.; McGregor, W.; Zait, K.; Schwing-Weill, M. J.; Barrett, G.; McKervey, M. A.; Marrs, D.; Seward, E. M. J. Chem. Soc., Perkin Trans. II 1995, 113.
- Yilmaz, M. Solution State Metal Complexes of Calixarenes and Polymeric Calixarene. In Handbook of Engineering Materials; Cheremisionoff, N. P., Ed.; Marcel Dekker, Inc: New York, 1997. [Google Scholar]
- Seangprasertkij, R.; Asfari, Z.; Arnaud, F.; Vicens, J. J. Org. Chem. 1994, 59, 1741. [CrossRef]
- Johnson, C. P.; Atwood, J. L.; Steed, J. W.; Bauer, C. B.; Rogers, R. D. Inorg. Chem. 1996, 35, 2602.
- Tamburini, S.; Tomasin, P.; Vigato, P. A.; Casnati, A.; Domiano, L. Inorganica Chimica Acta 1997, 254, 209.
- Yilmaz, M. Synth. React. Inorg. Met.-Org. Chem. 1998, 28, 1759. [CrossRef]
- Guo, T. D.; Zheng, Q. Y.; Yang, L. M.; Huang, Z. T. J. Inclusion Phenom. Macrosyclic Chem. 2000, 36, 327. [CrossRef]
- Verboom, W.; Durie, A.; Egberink, R. J. M.; Asfari, Z.; Reinhoudt, D. N. J. Org. Chem. 1992, 57, 1313. [CrossRef]
- Can, S.; Bekaroglu, O. J. Chem. Soc. Perkin Trans. I 1991, 3137.
- Deligoez, H.; Yilmaz, M. Synth. React. Inorg. Met-Org. Chem. 1997, 27, 391.
- Narag, K. K.; Shing, S. K.; Mishra, G. D. Synth. React. Inorg. Met-Org. Chem. 1996, 26, 191.
- Rashidi-Ranjbar, P.; Taghvaee-Gnajali, S.; Shaabani, B.; Akbari-Dilmaghani, K. Molecules 2000, 5, 941.
- Iwamoto, K.; Araki, K.; Shinkai, S. J. Org. Chem. 1991, 56, 4955. [CrossRef]
- McRae, E. G. J. Phys. Chem. 1975, 61. Paley, M. S.; Hariss, J. M. J. Org. Chem. 1989, 54, 3774. [CrossRef]
- Iwamoto, K.; Araki, K.; Shinkai, S. Tetrahedron 1991, 47, 4325.
- Sample Availability: Samples are available from the authors
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Alemi, A.A.; Shaabani, B.; Dilmaghani, K.A.; Ganjali, S.T. Synthesis and Characterization of Two New p-tert-Butylcalix[4]-arene Schiff Bases. Molecules 2001, 6, 417-423. https://doi.org/10.3390/60400417
Alemi AA, Shaabani B, Dilmaghani KA, Ganjali ST. Synthesis and Characterization of Two New p-tert-Butylcalix[4]-arene Schiff Bases. Molecules. 2001; 6(4):417-423. https://doi.org/10.3390/60400417
Chicago/Turabian StyleAlemi, Abdol Ali, Behrouz Shaabani, Karim Akbari Dilmaghani, and Saeed Taghvaee Ganjali. 2001. "Synthesis and Characterization of Two New p-tert-Butylcalix[4]-arene Schiff Bases" Molecules 6, no. 4: 417-423. https://doi.org/10.3390/60400417




