Alkylation of Zwitterionic Thiooxalic Acid Derivatives
Abstract
:Introduction
Results and Discussion

| Compd. | Formula | R1 | X | M.p. °C | Calculated/Found | |||
|---|---|---|---|---|---|---|---|---|
| (M.w.) | R2 | Yield % | %C | %H | %N | %S | ||
| 2a | C3H10IN5S | H | I | 119-120 | 13.09 | 3.66 | 25.45 | 11.65 |
| (275.11) | CH3 | 59 | 13.34 | 3.65 | 25.41 | 11.65 | ||
| 2b | C9H13BrN6O2S | H | Br | 92-94 | 30.95 | 3.75 | 24.06 | 9.18 |
| (349.20) | 4-NO2C6H4CH2 | 72 | 30.74 | 3.73 | 23.51 | 9.13 | ||
| 3 | C11H17BrN6O2S | CH3 | Br | 87-90 | 35.02 | 4.54 | 22.28 | 8.50 |
| (377.26) | 4-NO2C6H4CH2 | 92 | 35.10 | 4.62 | 22.19 | 8.38 | ||
| 4a | C15H18IN5S | C6H5 | I | 196-200 | 42.16 | 4.25 | 16.39 | 7.50 |
| (427.30) | CH3 | 62 | 41.99 | 4.35 | 16.35 | 7.41 | ||
| 4b | C16H20IN5S | C6H5 | I | 195-200 | 43.54 | 4.57 | 15.87 | 7.26 |
| (441.33) | C2H5 | 65 | 43.44 | 4.51 | 15.99 | 7.17 | ||
| 4c | C24H36BrN5S | C6H5 | Br | 75-80 | 56.91 | 7.16 | 13.83 | 6.33 |
| (506.54) | CH3(CH2)9 | 78 | 56.76 | 7.14 | 13.30 | 6.15 | ||
| 4d | C21H22BrN5S | C6H5 | Br | 205-209 | 55.26 | 4.86 | 15.34 | 7.02 |
| (456.41) | C6H5CH2 | 40 | 55.30 | 4.96 | 15.34 | 7.03 | ||
| 4e | C21H21BrN6O2S | C6H5 | Br | 140-145 | 50.31 | 4.22 | 16.76 | 6.39 |
| (501.40) | 4-NO2C6H4CH2 | 67 | 49.42 | 4.54 | 16.50 | 6.34 | ||
| Compound | 4b | 4d |
| Empirical formula | C16H20IN5S | C21H22BrN5S |
| Formula weight | 441.33 | 456.41 |
| Crystal system | .................... Monoclinic............................ | |
| Space group | C2/c | P21/n |
| Unit cell dimensions [Å] | a = 22.963(5) | 13.391(3) |
| b = 13.236(3) | 10.928(3) | |
| c = 16.537(3) | 15.504(3) | |
| β = 132.44(3)° | β = 111.03(2)° | |
| Volume [Å3] | 3709.3(13) | 2117.7(9) |
| ρ (calculated) [g cm-3] | 1.581 | 1.432 |
| Z | 8 | 4 |
| F (000) | 1760 | 936 |
| μ(Mo-Kα) [ mm-1 ] | 1.845 | 2.056 |
| Crystal size [mm] | 0.52 x 0.44 x 0.24 | 0.52 x 0.39 x 0.30 |
| 2Θ range | 3.9/44 | 4.68/45.98 |
| hkl range | -24/1, -1/13, -13/17 | -1/14, -12/1, -17/16 |
| Measured refl. | 2750 | 3747 |
| Unique refl. | 2271 | 2910 |
| Observed refl. | 1948 | 2231 |
| Completeness to Θ = 22.00° | 99.9% | 99.2% |
| Data / restraints / parameters | 2271 / 0 / 228 | 2910 / 0 / 257 |
| Min. transm. | 0.59421 | 0.12686 |
| Max. transm. | 0.83244 | 0.16969 |
| R1 for observed refl. | 0.0350 | 0.0433 |
| R1 for all refl. | 0.0417 | 0.0643 |
| wR2 for all refl. | 0.0930 | 0.1097 |
| GoF on F2 | 1.022 | 1.009 |
| ρ (max/min) [e.Å-3 ] | 0.374 / -0.442 | 0.319 / -0.426 |
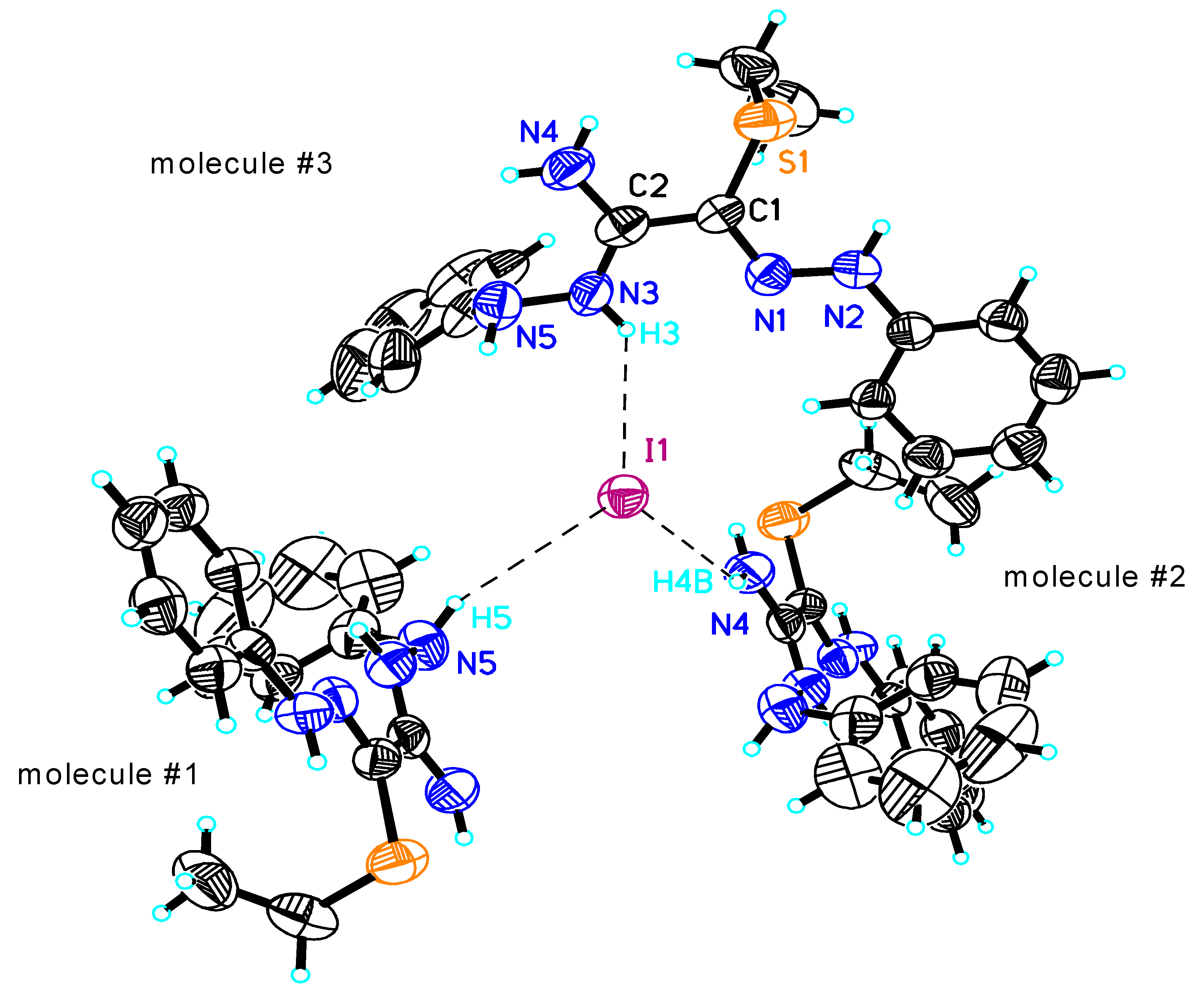
| S(1)-C(1) | 1.763(5) | N(4)-C(2) | 1.315(7) | H(2)...S(1) | 2.56 |
| N(1)-C(1) | 1.302(6) | C(1)-C(2) | 1.462(7) | N(3)-H(3)...N(1) | 2.680(6) |
| N(1)-N(2) | 1.313(6) | N(4)-H(4A)...S(1) | 3.019(7) | H(3)...N(1) | 2.37 |
| N(3)-C(2) | 1.311(7) | H(4A)...S(1) | 2.59 | N(3)-C(2)-N(4) | 120.5(6) |
| N(3)-N(5) | 1.408(7) | N(2)-H(2)...S(1) | 2.959(5) | N(3)-C(2)-C(1) | 120.5(5) |
| N(4)-C(2)-C(1) | 119.0(6) | N(4)-H(4B)...I(1)#2 | 3.539(6) | C(2)-C(1)-S(1) | 120.6(4) |
| N(2)-N(1)-C(1)-C(2) | -177.8(4) | H(4B)...I(1)#2 | 2.96 | N(5)-N(3)-C(2)-C(1) | 178.0(5) |
| N(2)-N(1)-C(1)-S(1) | 1.7(6) | N(3)-H(3)...I(1)#3 | 3.518(5) | N(1)-C(1)-C(2)-N(3) | -3.1(7) |
| N(5)-N(3)-C(2)-N(4) | -3.7(8) | H(3)...I(1)#3 | 2.83 | S(1)-C(1)-C(2)-N(3) | 177.4(4) |
| N(4)-H(4B)...N(5) | 2.678(9) | C(1)-N(1)-N(2) | 119.5(4) | N(1)-C(1)-C(2)-N(4) | 178.7(5) |
| H(4B)...N(5) | 2.34 | C(2)-N(3)-N(5) | 119.6(5) | S(1)-C(1)-C(2)-N(4) | - 0.9(6) |
| N(5)-H(5)...I(1)#1 | 3.989(6) | N(1)-C(1)-C(2) | 115.1(4) | ||
| H(5)...I(1)#1 | 3.21 | N(1)-C(1)-S(1) | 124.3(4) |
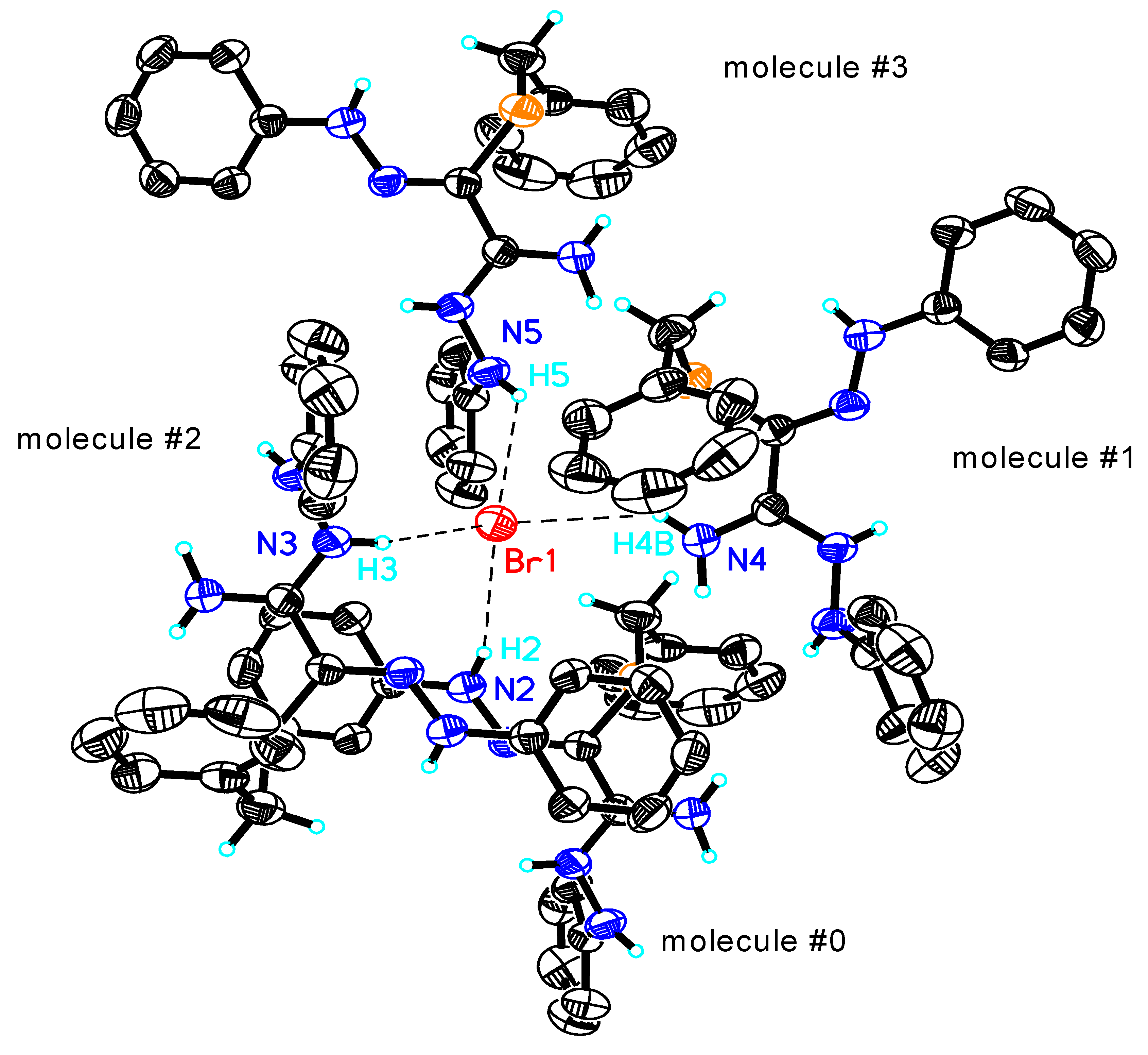
| S(1)-C(1) | 1.750(4) | H(4B)...Br(1)#1 | 2.77 | N(4)-C(2)-N(3) | 120.1(4) |
| N(4)-C(2) | 1.297(6) | N(2)-H(2)...Br(1) | 3.683(4) | N(4)-C(2)-C(1) | 121.5(4) |
| N(1)-C(1) | 1.310(5) | H(2)...Br(1) | 2.89 | N(3)-C(2)-C(1) | 118.3(4) |
| N(1)-N(2) | 1.313(5) | N(3)-H(3)...Br(1)#2 | 3.343(4) | N(1)-C(1)-C(2)-N(4) | 159.2(4) |
| N(3)-C(2) | 1.327(6) | H(3)...Br(1)#2 | 2.51 | S(1)-C(1)-C(2)-N(4) | -16.6(6) |
| N(3)-N(5) | 1.402(5) | N(5)-H(5)...Br(1)#3 | 3.470(4) | N(1)-C(1)-C(2)-N(3) | -17.9(6) |
| C(1)-C(2) | 1.466(6) | H(5)...Br(1)#3 | 2.83 | S(1)-C(1)-C(2)-N(3) | 166.2(3) |
| N(4)-H(4A)...S(1)#1 | 3.353(4) | C(1)-N(1)-N(2) | 120.7(4) | N(2)-N(1)-C(1)-C(2) | -179.5(4) |
| H(4A)...S(1)#1 | 2.75 | C(2)-N(3)-N(5) | 119.7(4) | N(2)-N(1)-C(1)-S(1) | -4.0(6) |
| N(4)-H(4B)...S(1) | 3.061(4) | N(1)-C(1)-C(2) | 113.8(4) | N(5)-N(3)-C(2)-N(4) | 1.1(7) |
| H(4B)...S(1) | 2.64 | N(1)-C(1)-S(1) | 126.4(3) | N(5)-N(3)-C(2)-C(1) | 178.3(4) |
| N(4)-H(4B)...Br(1)#1 | 3.454(4) | C(2)-C(1)-S(1) | 119.6(3) |
Experimental
General
Method A: Preparation of halides 2
S-Methyl-thiooxal-1-hydrazono-2-amidrazonium iodide (2a)

S-(4-Nitrobenzyl)thiooxal-1-hydrazono-2-amidrazonium bromide (2b)

Method B: Preparation of halides 3 and 4
S-(4-Nitrobenzyl)thiooxal-1-(2-methylhydrazono)-2-(2-methylamidrazonium)bromide (3)

S-Methyl-thiooxal-1-(2-phenylhydrazono)-2-(2-phenylamidrazonium)iodide (4a)

S-Ethyl-thiooxal-1-(2-phenylhydrazono)-2-(2-phenylamidrazonium)iodide (4b)

S-Decyl-thiooxal-1-(2-phenylhydrazono)-2-(2-phenylamidrazonium)bromide (4c)

S-Benzyl-thiooxal-1-(2-phenylhydrazono)-2-(2-phenylamidrazonium)bromide (4d)

S-(4-Nitrobenzyl)thiooxal-1-(2-phenylhydrazono)-2-(2-phenylamidrazonium)bromide (4e)

X-Ray structure determinations
Acknowledgments
References and Notes
- Bauer, W.; Kühlein, K. Methoden der organischen Chemie (Houben-Weyl): Carbonsäuren und Carbonsäure-Derivate; Falbe, J., Ed.; Georg Thieme Verlag: Stuttgart, New York, 1985; Vol. E5, Part 2; pp. 1263, 1300. [Google Scholar]
- Jensen, K. A.; Pedersen, C. Studies of Thioacids and Their Derivatives. VI. Formation of Thiadiazoles and Tetrazines in the Preparation of Thiohydrazides. Acta Chem. Scand. A 1961, 15, 1124–1129. [Google Scholar] [CrossRef]
- Dehne, H.; Scheunemann, A.; Michalik, M.; Hartung, H.; Heinemann, F.; Kibbel, H. U. Thiooxalsäure-2-amid-1-hydrazid-2-hydrazon: Eine neue zwitterionische Verbindung. Phosphorus, Sulfur Silicon Relat.Elem. 1994, 86, 177–179. [Google Scholar] [CrossRef]
- Drexler, K.; Dehne, H.; Reinke, H.; Michalik, M. Reactions of Sodium Cyanodithioformate with Monosubstituted Hydrazines. Liebigs Ann./Recueil 1997, 269–271. [Google Scholar] [CrossRef]
- Drexler, K.; Dehne, H.; Reinke, H.; Michalik, M. Reactions of Zwitterionic Thiooxamic Acid Derivatives with Alkyl Bromoacetates. Sulfur Lett. 1998, 21, 163–177. [Google Scholar]
- Dehne, H.; Drexler, K.; Martens, K.; Reinke, H.; Michalik, M. Zwitterionic Thiooxamic Acid Derivatives as Efficient Building Blocks for Biheterocycles. Sulfur Lett. 2000, 24, 29–37. [Google Scholar]
- Sample Availability: Samples of compounds 1a-c, 2a, 2b and 4b are available from MDPI.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Martens, K.; Scheunemann, A.; Drexler, K.; Dehne, H.; Reinke, H.; Michalik, M. Alkylation of Zwitterionic Thiooxalic Acid Derivatives. Molecules 2001, 6, 540-550. https://doi.org/10.3390/60600540
Martens K, Scheunemann A, Drexler K, Dehne H, Reinke H, Michalik M. Alkylation of Zwitterionic Thiooxalic Acid Derivatives. Molecules. 2001; 6(6):540-550. https://doi.org/10.3390/60600540
Chicago/Turabian StyleMartens, Klaus, Anke Scheunemann, Kerstin Drexler, Heinz Dehne, Helmut Reinke, and Manfred Michalik. 2001. "Alkylation of Zwitterionic Thiooxalic Acid Derivatives" Molecules 6, no. 6: 540-550. https://doi.org/10.3390/60600540





